
Physiology News Magazine
A time to fast, a time to feast
The concept of chrononutrition
Features
A time to fast, a time to feast
The concept of chrononutrition
Features
Henrik Oster, Lichtenberg Endowed Chair of Neurobiology, Institute of Neurobiology, Centre of Brain, Behaviour & Metabolism, University of Lübeck, Germany
https://doi.org/10.36866/pn.113.40
The concept of chrononutrition
In the Bible it is written, “There is a time for everything, and a season for every activity under the heavens” (Book of Ecclesiastes). It goes on listing various timed activities, from being born and dying to war and peace. There is no mention of eating and fasting, though, which is remarkable when considering that (a) many religions pay special attention to dietary rules including its timing, and (b) that both old folk wisdom and recent research data suggest that the timing of meal intakes may have profound effects on well-being and health. The latter is linked to our internal clock system.
Circadian clocks and rhythms
During the course of evolution, most species on this planet have developed internal timing systems in order to track the course of the 24-hour day under noisy environmental conditions. These circadian (from Latin circa diem – “around the day”) clocks coordinate physiology and behaviour and enable an organism to anticipate daily recurring events and demands. In humans, circadian clocks control daily rhythms in a wide array of biological functions, from sleep-wake behaviour to cognitive processes and immunity.
At the molecular level, circadian clocks are based on transcriptional-translational feedback loops comprising a set of clock genes and their corresponding proteins (Takahashi, 2017). Two transcriptional activators, CLOCK (circadian locomotor output cycles kaput) and BMAL1 (brain and muscle ARNT-like 1/ARNTL) promote transcription of three PERIOD (PER1-3) and two CRYPTOCHROME (CRY1/2) genes during the day. PER and CRY proteins form dimers to translocate back into the nucleus during the night, inhibiting CLOCK/BMAL1 activity – thus shutting down their own production (Fig. 1).
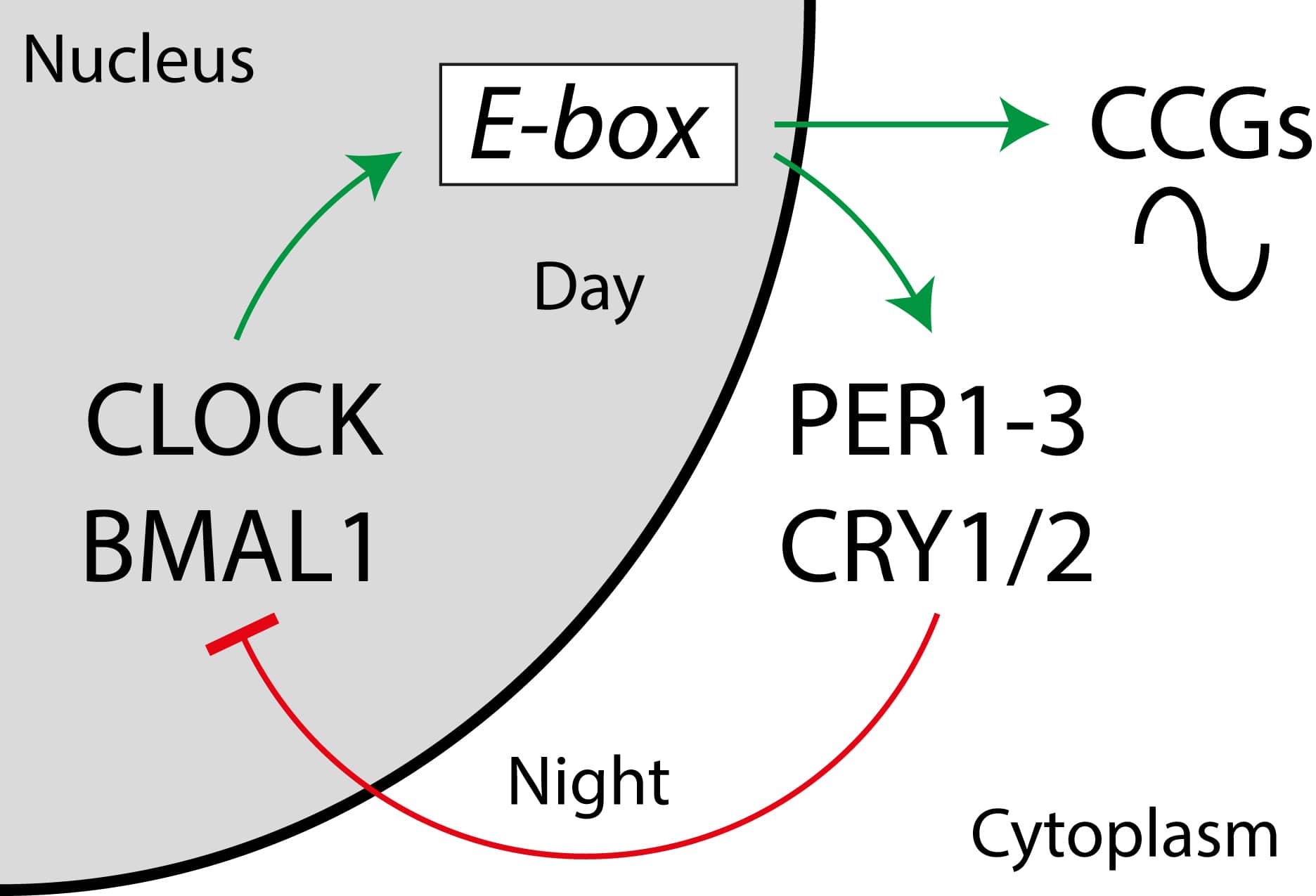
Towards the end of the night, PER/CRY complexes are degraded, freeing CLOCK/BMAL1 from repression and reinitiating the next circadian cycle. Further feedback loops stabilise this core clock oscillator. Importantly, besides PERs and CRYs, large numbers of clock-controlled genes show rhythmic regulation of transcription, thereby translating clock-time information into physiological functions. It is estimated that 5-10 % of active genes in each tissue are under circadian control. In mammals, a central circadian pacemaker resides in the hypothalamic suprachiasmatic nucleus (SCN), coordinating clocks in all tissues of the body with each other and with the external light-dark cycle.
Clock disruption and metabolic homeostasis
An important target of the circadian clock is energy metabolism (Bass, 2012). Circadian clocks in central regulatory circuits control appetite and energy expenditure, while clocks in peripheral metabolic tissues modulate all levels of energy conversion – from food digestion and nutrient absorption in the intestinal tract to energy storage as glycogen in liver and muscle and as lipid droplets in adipose tissues. Further, systemic regulators of energy metabolism such as insulin and leptin and the autonomic nervous system are regulated by circadian clocks.
This intricate network of circadian checkpoints allows for a tight regulation of metabolic functions along the course of the day, optimising energy efficiency and improving evolutionary fitness under natural conditions (Fig. 2).
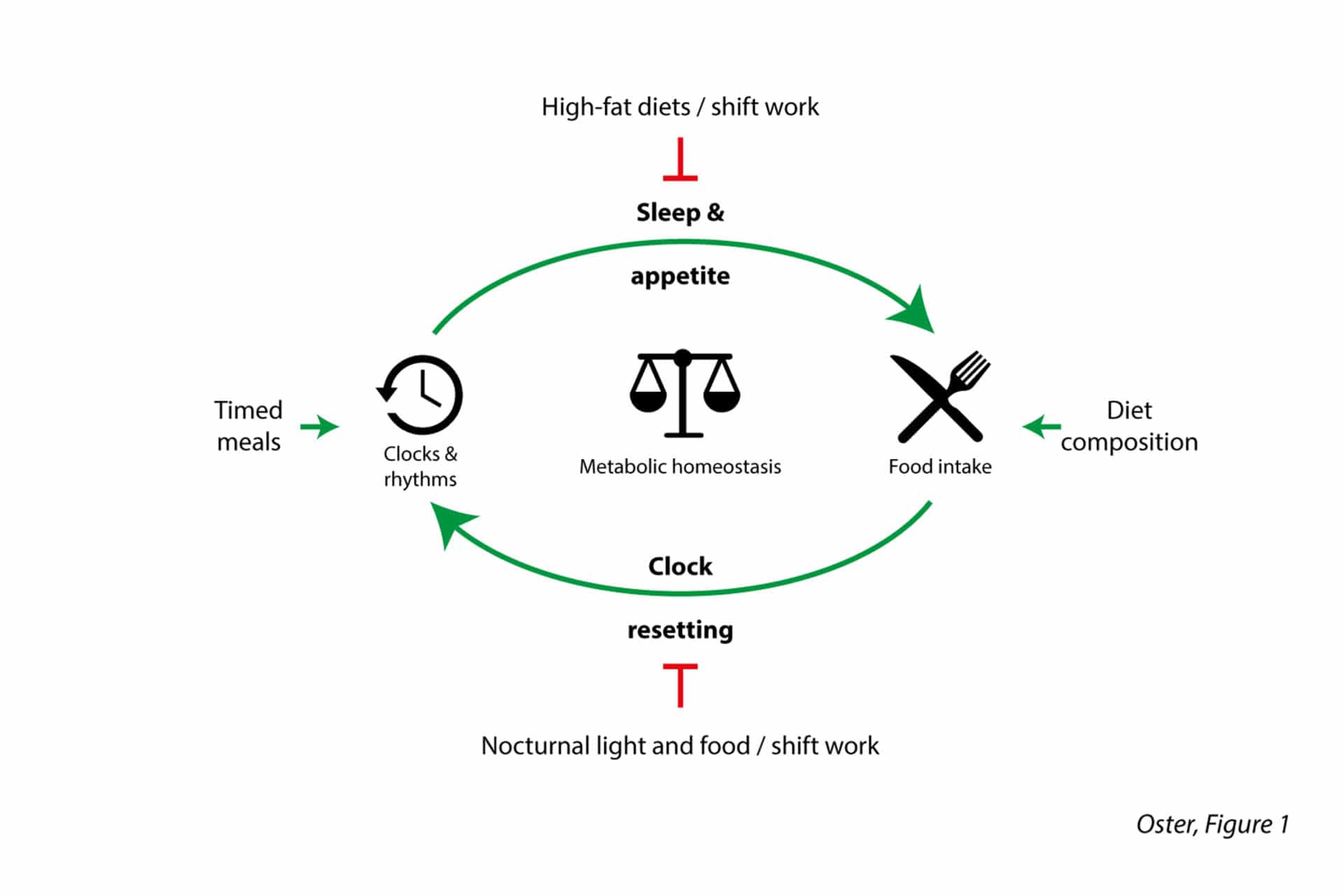
In our modern societies of food abundance and 24-hour demands, however, this ancient system frequently reaches its limits. More and more people work shift schedules with unnatural sleep and meal conditions, while even non-shift workers disrupt natural timing cues (or Zeitgeber – from German “time giver”) of their circadian metabolism by artificial lighting or transcontinental air travel. In shift workers, the temporal coordination of physiological processes is altered, rendering them more vulnerable to a whole range of disorders, from obesity to depression and even cancer. Chronodisruptive risk factors for shift workers
include nocturnal light exposure (acting through the SCN), mistimed meal intake (acting through peripheral clocks), sleep loss and disruption, and the alteration of endocrine signals such as cortisol and melatonin. In consequence, virtually all major metabolic diseases are more prevalent in shift workers than in non-shift workers. As two examples, the risk for type-2 diabetes is up by more than 50 % in shift workers, for hypertension by about 30 % (Lemmer & Oster, 2018).
Circadian regulation of appetite
Energy homeostasis is defined as the balance between energy intake and expenditure. Already at the level of appetite regulation, circadian clocks play an important role. One principle regulator of food intake is sleep. Quite obviously, during sleep eating is impossible. At the same time, during the circadian rest phase (i.e. the night in humans and the day in nocturnal rodents) appetite is suppressed – with further inhibition during actual sleep. While clocks in hypothalamic nuclei involved in energy balance affect the homeostatic aspects of appetite regulation, central reward pathways have been implicated in food choice and the selection of different nutrient sources. Again, circadian clocks are involved in regulating these hedonic aspects of appetite. This represents a major target for physiologists, pharmacologists, and nutrition scientists considering that in an environment of abundant food availability and constant psychological manipulation by advertisements, hedonic appetite drive may become the determining factor of energy homeostasis and metabolic health. Recent data suggest that dopaminergic reward circuits may be under circadian control. Dysregulation of clocks in these circuits may affect externalised eating behaviour and promote food overconsumption – but also other aspects of addictive behaviours (Blancas-Velazquez et al.).
Food as a Zeitgeber
Not only do clocks regulate appetite and food intake, the timing of meals and meal composition may feed back on circadian clock function. Rodent experiments have shown that clock gene activity rhythms in peripheral tissues such as the liver and adipose tissues respond rapidly to changes in the daily feeding schedule. In extreme cases such as rest phase-only eating, this results in an uncoupling of peripheral clocks and rhythms from the SCN pacemaker, leading to a state of internal desynchrony (Landgraf et al., 2017). Factors involved in this uncoupling are insulin and incretin peptides such as glucagon-like peptide 1 (GLP-1) and oxyntomodulin. The nature of nutrients may further affect the circadian system. Under a normal chow diet (ca. 10 % fat), mice eat more than 80 % of their food during the (active) night period. If they are shifted to a high-fat diet (40-60 % fat), they increasingly continue feeding during the day (up to 40 %), resembling feeding behaviour in mice with genetically ablated clock function. In mice and in humans, such rest phase eating has been associated with increased body weight. Thus, high-lipid diets can lead to a vicious cycle – promoting rest-phase eating and disrupting circadian clock rhythms, both of which promote overeating and weight gain. It has been suggested that this phenomenon itself may explain most of the metabolic consequences of shift work, but may also impact on other aspects of obesity such as systemic inflammation or addictive behaviours.
Time-restricted (interval) diets
Considering the profound effect of meal timing on circadian function, researchers like Satchin Panda from the Salk Institute in La Jolla, USA had the idea to turn this around and use meal timing as an intervention to positively affect energy homeostasis and other disorders associated with circadian disruption (Longo & Panda, 2016). In C57BL/6 mice, a high-fat diet provided throughout the day leads to a massive increase in body weight – up to 100 % in just 10 weeks! If, however, access to food is restricted to just 8 hours during the active phase, animals stay slim (about 10 % weight gain) although energy intake is comparable to that of their ad-libitum fed mates. Similarly, in humans, long-term monitoring of meal intake patterns revealed that most of us regularly eat during a time window of 18 hours starting at around 6 am. When subjects were asked to reduce this window to 10 hours, they lost around 5 % body weight within 4 months without any calorie restrictions (Gill & Panda, 2015). These data suggest that meal (or rather fasting) timing may be an important regulator of energy homeostasis and that a resetting of the circadian clock system may underlie the positive effect of interval fasting on body weight regulation and subjective well-being. Ongoing studies aim at determining other metabolic and non-metabolic targets of timed meal patterning and to what extent the composition of different meals may further affect these functions. While few conclusions can be drawn at this point, it has already been shown that increased cereal/dairy intake during breakfast is associated with better cardiometabolic health. Likewise, early lunch times seem to be a positive predictive factor for weight loss therapy outcomes.
Conclusion
Accumulating evidence suggests an extensive crosstalk between circadian clocks and rhythms and the regulation of energy metabolism. On one hand, synchrony amongst the different components of the circadian network promotes metabolic homeostasis and health. On the other hand, meal timing and nutrient composition affect circadian regulation, thus impacting not only on metabolic functions, but also on further target systems of the circadian clock. The dissection of the molecular underpinnings of this crosstalk will provide interesting therapeutic targets for the prevention and treatment of metabolic disorders in a globalised 24-hour society.
Acknowledgements
HO is a Lichtenberg fellow of the Volkswagen Foundation.
References
Bass J (2012). Circadian topology of metabolism. Nature 491(7424), 348-356.
Blancas-Velazquez A, Mendoza J, Garcia AN et al. (2017). Diet-induced obesity and circadian disruption of feeding behavior. Frontiers in Neuroscience 11, 23.
Ecclesiastes 3:1
Gill S, Panda S (2015). A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metabolism 22(5), 789-798.
Landgraf D, Neumann AM, Oster H (2017). Circadian clock-gastrointestinal peptide interaction in peripheral tissues and the brain. Best Practice & Research:
Clinical Endocrinology & Metabolism 31(6), 561-571.
Lemmer B, Oster H (2018). The role of circadian rhythms in the hypertension of diabetes mellitus and the metabolic syndrome. Current Hypertension Reports 20(5), 43.
Longo VD, Panda S (2016). Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metabolism 23(6), 1048-1059.
Takahashi JS (2017). Transcriptional architecture of the mammalian circadian clock. Nature Reviews Genetics 18(3), 164-179.
