Physiology News Magazine
The highs of endocannabinoid physiology
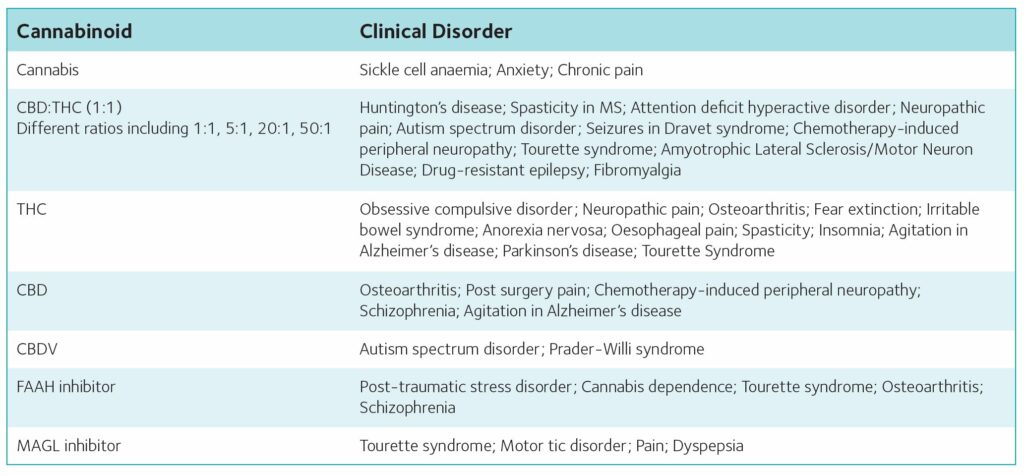
Currently licenced cannabinoids and targeting of the endocannabinoid system
Features
The highs of endocannabinoid physiology
Currently licenced cannabinoids and targeting of the endocannabinoid system
Features
https://doi.org/10.36866/pn.124.23
Dr Aoife M Thornton, Institute of Neuroscience, Trinity College Dublin, Ireland
Dr Michelle Roche, National University of Ireland Galway, Ireland

The marijuana plant, Cannabis sativa, has been used for medicinal, recreational, and religious purposes for millennia. The Irish-born physician Sir William O’Shaughnessy is credited with introducing cannabis-based medicine to the western world in the 1800s after noting significant medicinal effects of cannabis for conditions such as epilepsy and pain while working in India. Reports from the 1840s suggest that Queen Victoria used cannabis for the relief of menstrual pain and morning sickness. However, the medicinal use of cannabis declined in the 1900s due to the social and political concerns, emergence of legalisation prohibiting the recreational use of cannabis and the development of more selective therapeutics.
Interest in the medicinal effects of cannabis re-emerged when the primary psychoactive component of the cannabis plant, Δ9- tetrahydrocannabinol (THC) was isolated in the early 1960s by Professor Raphael Mechoulam, now regarded as the godfather of modern-day cannabinoid research. Shortly afterwards, the non-psychoactive phytocannabinoid cannabidiol (CBD) was isolated. However, it was a further 30 years before the receptors that bind THC were identified in the early 1990s, namely cannabinoid (CB)1 receptor and CB2 receptor. While both receptors are located throughout the body, CB1 receptors are highly expressed in the central nervous system – activation of which by THC is responsible for the psychoactive effects associated with cannabis. In comparison, CB2 receptors are highly expressed on cells and tissues of the immune system – activation of which elicit potent immunomodulatory effects.
Shortly after the discovery of the CB receptors, the endogenous ligands that bind to these receptors were identified and named the endocannabinoids – the best characterised of which are anandamide and 2-arachidonoylglycerol (2-AG). The endocannabinoids are synthesised on demand from membrane phospholipids by a range of enzymes, in response to activity dependent depolarisation and the subsequent increase in intracellular calcium levels (Fig.1). Following release, the endocannabinoids bind to the Gi/ o-coupled CB receptors, which are negatively coupled to adenylyl cyclase and positively coupled to mitogen-activated protein kinase, resulting in downstream changes in gene expression and cellular activity. In addition, endocannabinoids act as retrograde inhibitory neurotransmitters in the central nervous system, where binding to presynaptic CB1 receptors results in the inhibition of N- and P/Q-type voltage-gated calcium channels and the induction of inwardly rectifying potassium currents with the resultant inhibition of neurotransmitter release (Fig.1). In addition to CB1 and CB2 receptors, endocannabinoids also have an affinity for, and activity at, several non-cannabinoid receptors including transient receptor potential vanilloid 1, the nuclear family of peroxisome proliferator- activated receptors and a number of orphan G-protein coupled receptors, GPR55, GPR18 and GPR119. Catabolism of endocannabinoid mainly occurs via the enzymes fatty acid amide hydrolase (FAAH) for anandamide, and monoacylglycerol lipase (MAGL) for 2-AG.
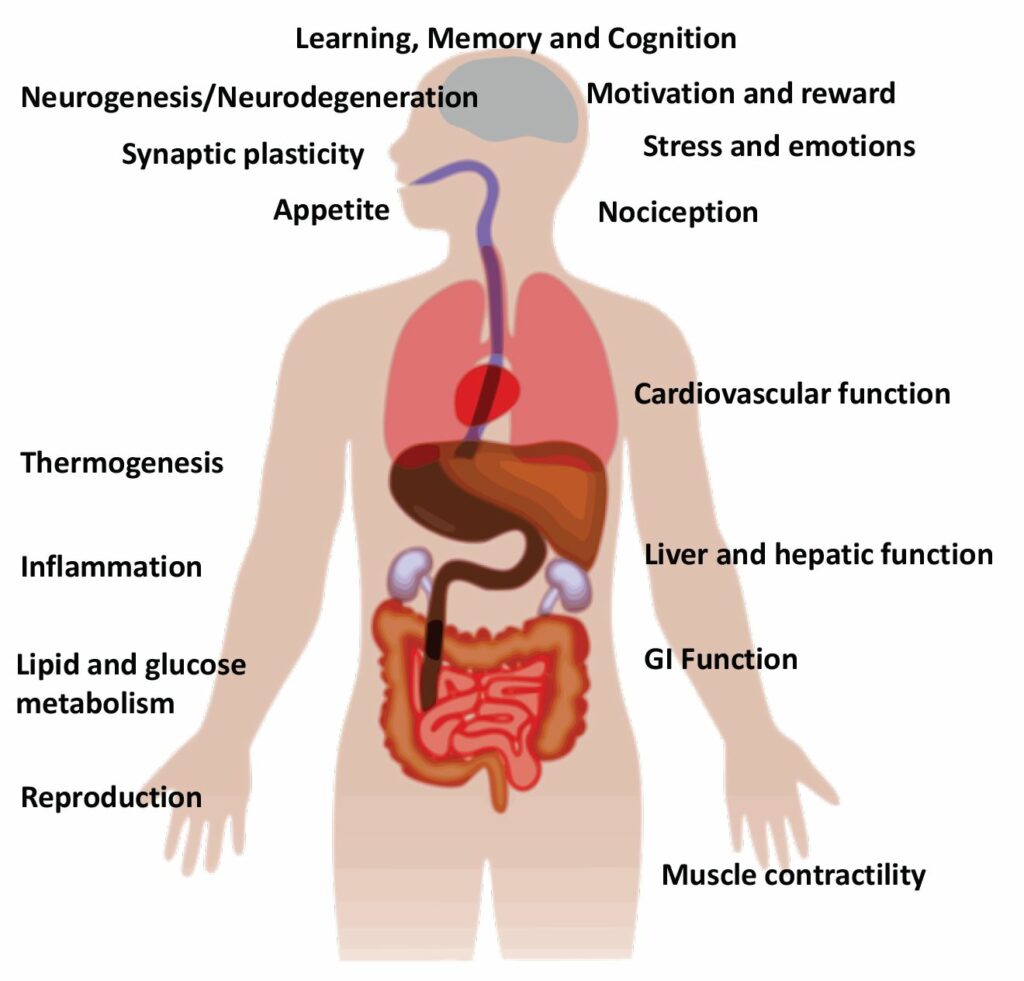
The endocannabinoid system is responsible for mediating and modulating a wide array of physiological functions throughout the body, including emotion, pain, immune function, reproduction, cardiovascular activity, and gastrointestinal function (Fig.2). As such, it is not surprising that there has been an explosion of research over the past 20 years examining the effects of cannabis, phytocannabinoids (most notably THC and CBD), and endocannabinoid system modulators on various medical conditions including cancer, chronic pain, and obesity.
Currently licenced cannabis-based medicines, synthetic cannabinoids and clinical trials
Political, public and patient support has resulted in regulatory changes in several countries including the UK, US and several European countries, permitting the use of cannabis for specific medical conditions such as multiple sclerosis, retractable epilepsy and chronic pain. While these changes have benefited many patients, the content of phytocannabinoids in the cannabis plant can greatly differ between batches and strains.
Furthermore, the cannabis plant also contains a large number of terpenes, flavonoids and other compounds, which, themselves, may be pharmacologically active. In comparison, cannabis-based medicines are registered medicinal cannabis extracts, with well-defined and standardised THC and/or CBD contents. Such medicines have been licenced for the treatment of spasticity associated with multiple sclerosis, epileptic seizures associated with Lennox-Gastaut syndrome or Dravet syndrome and chemotherapy-induced nausea and vomiting (see Table 1).

In addition to cannabis-based medicines, endocannabinoid system modulators have also shown therapeutic promise and a large number of clinical trials are currently underway into the effects in various medical conditions (Table 2). The CB1 antagonist/inverse agonist Rimonabant® was briefly licenced for the treatment of obesity in 2006. Although a very effective treatment, a number of patients developed psychiatric side effects, most notably depression, resulting in withdrawal of its licence. However, research on the anti-obesity effects of CB1 antagonists continues with current research focused on neutral and peripherally restricted CB1 antagonists, which are devoid of psychiatric side effects. Inhibiting the catabolic enzyme FAAH specifically increases AEA and related N-acylethanolamine levels in areas involved in specific physiological responses. FAAH inhibitors have demonstrated therapeutic efficacy in several preclinical models, without inducing psychoactive effects associated with direct CB1 receptor activation. Furthermore clinical trials have shown that FAAH inhibitors are well tolerated and effective in the treatment of cannabis withdrawal and dependence, and pain in osteoarthritis. However, as with all pharmacological agents, selectivity is important and in 2016, a clinical trial of a FAAH inhibitor was terminated following the death of a male volunteer and the brain injury of four others. Subsequent investigations found that these adverse effects were not due to FAAH inhibition but rather due to off-target effects of this specific compound. Such effects have not been observed with subsequent selective FAAH inhibitors, and as such these compounds have the potential to provide novel therapeutic strategies for a number of disorders, with several clinical trials underway examining efficacy in treatment of post-traumatic stress disorder, Tourette syndrome and schizophrenia.
Therapeutic potential of cannabinoids and endocannabinoid modulators
It is not possible to provide a complete overview of all the physiological effects of the endocannabinoid system or pathophysiological conditions that may benefit from cannabis-based medicines or endocannabinoid system modulators (see recent review for overview (Lowe et al., 2021)). As such, we will provide a brief overview of some key recent research endeavours in the field relating to neuroscience and neurological conditions.
Acute and chronic pain
The analgesic effects of cannabis were some of the first recorded medicinal effects, and as such it is unsurprising that cannabis, cannabis-based medicines, and modulators of the endocannabinoid system have received considerable interest as treatments for pain (i.e. analgesics). This has been further fuelled by recent endeavours to identify non-opioid alternatives for pain management and the licencing of medical marijuana for the treatment of certain pain conditions in several countries including the UK and several EU countries. Components of the endocannabinoid system are located at all levels of the pain pathway from peripheral nociceptors, dorsal horn spinal cord neurons, and in specific brain regions involved in ascending and descending pain modulation. Cannabinoids and enhancing endocannabinoid tone have been shown to reduce nociceptive transmission at all of these levels. Furthermore, increasing evidence also indicates a key role for the endocannabinoid system in glial cells in modulating nociception. The high comorbidity of mood disorders and chronic pain is widely recognised, with up to 70% of chronic pain patients experiencing comorbid anxiety and/or depression, and increasing preclinical evidence from our group and others indicates that cannabinoids and endocannabinoid modulators may provide a novel therapeutic target for patients that may exhibit co-morbid depression and chronic pain (Fitzgibbon et al., 2015).
To distil the wealth of data, both clinical and preclinical, in various models and conditions, both the European Pain Federation (EFIC) and the International Association for the Study of Pain (IASP) established task forces on the use of cannabis, cannabinoids and cannabis-based medicines for pain management. Systematic reviews and meta-analysis indicate considerable support for cannabinoid-induced analgesia, in particular for treatment of neuropathic pain (Hauser et al., 2018; Soliman et al., 2021). EFIC recommend that “therapy with cannabis-based medicines should only be considered by experienced clinicians as part of a multidisciplinary treatment and preferably as adjunctive medication if guideline-recommended first- and second-line therapies have not provided sufficient efficacy or tolerability”. However, it was acknowledged that translating the wealth of preclinical findings to the clinic remains challenging and currently “IASP does not currently endorse general use of cannabis and cannabinoids for pain relief but rather recognizes the pressing need for further studies to fill the research gap”.
Neuroinflammatory and neurodegenerative disorders
Uncontrolled systemic and central immune responses have been proposed to underlie the pathophysiology and exacerbation of a host of neurological and psychiatric conditions. A wealth of evidence over the past two decades has demonstrated potent immunomodulatory effects of cannabis, cannabis-based medicines and endocannabinoid modulators, with effects dependent on the compound investigated, time of administration and condition under investigation. Glial activation and neuroinflammation is associated with increased expression of CB2 receptors.
As such several research groups have examined the therapeutic potential of CB2 receptor agonists or enhancing endogenous endocannabinoid tone in various preclinical models, noting significant beneficial effects both on neuroinflammation and neuroprotection (Henry et al., 2016; Kelly et al., 2020). A number of clinical trials are ongoing examining the effects of cannabinoids in neurodegenerative disorders including Huntington’s disease, Alzheimer’s disease and Parkinson’s disease (Table 2).
Autism spectrum disorder symptoms
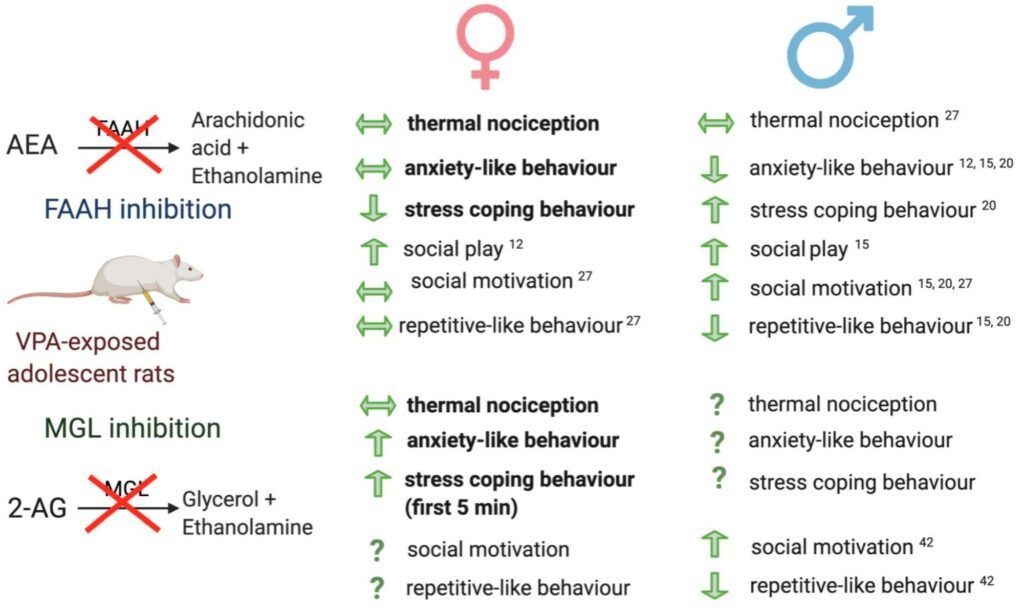
Anecdotal evidence reporting autism spectrum disorder (ASD) symptom improvement following the intake of cannabis or medical marijuana has resulted in the establishment of numerous advocate groups. While the precise mechanism mediating the potential beneficial effects of cannabis or medical marijuana remain to be uncovered, alterations in CB2 receptor expression and circulating levels of the endocannabinoids have been reported in ASD individuals (Aran et al., 2019). The endocannabinoid system is well recognised to play a key role in mediating and modulating social responding, repetitive behaviour and emotion, alterations that represent core behavioural changes in ASD individuals. Data from our lab and others have demonstrated alterations in endocannabinoid system and beneficial effects of cannabinoids or enhancing endocannabinoid tone on core ASD behavioural alterations in environmental and genetic models of ASD (Fig. 3) (Zamberletti et al., 2017; Thornton et al., 2021). A number of recent clinical trials have demonstrated beneficial effects of phytocannabinoids for the treatment of specific ASD symptoms such as self- injurious behaviour, hyperactivity, sleep problems and anxiety (Fusar-Poli et al., 2020; Silva et al., 2021), although there is wide recognition that randomised, double blind, placebo-controlled clinical trials are necessary to clarify and further support findings. Currently, there are four clinical trials registered examining the efficacy and safety of CBD, CBDV and CBD:THC (1:1 ratio) in ASD.
Future directions
The endocannabinoid system is present in every cell of the body and thus modulates a wide array of physiological responses. New technological and methodological advances in the measurement of endocannabinoids, site- and circuit- specific genetic and pharmacological modulation and the development of more clinically relevant preclinical models, will enable a greater understanding of the role of the endocannabinoid system and its effects on physiological processes.
The need for rigorous, balanced, and well-controlled clinical trials is imperative to determine if the wealth of preclinical data can be translated to the clinic and ultimately provide new therapeutics. Accordingly, at the time of writing this article there are over 450 registered clinical trials examining the efficacy and safety of cannabis, cannabis-based medicines or endocannabinoid modulators for various medical conditions. Harnessing the potential of the endocannabinoid system without inducing psychoactive or global immunosuppressive effects will be key to the success of these products. As such CBD, which has no affinity for the cannabinoid receptors, peripherally restricted CB1 receptors, and FAAH or MAGL inhibitors, which increase endocannabinoid tone, may be particularly beneficial. Future studies that take into account the stratification of patients, the inclusion rather than exclusion of patients with co-morbidities, and examination of effects of sex and age, will further enhance our understanding of the system and its potential as a therapeutic target.
References
Aran A et al. (2019). Lower circulating endocannabinoid levels in children with autism spectrum disorder. Molecular Autism 10,2. https://dx.doi.org/10.1186/s13229-019-0256-6
Cannabis IPTFo, Cannabinoid A (2021). International Association for the Study of Pain Presidential Task Force on Cannabis and Cannabinoid Analgesia position statement. Pain 162,S1-S2. https://dx.doi.org/10.1097/j.pain.0000000000002265
Finn DP et al. (2021). Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain 162,S5-S25. https://dx.doi.org/10.1097/j.pain.0000000000002268
Fitzgibbon M et al. (2015). High times for painful blues: the endocannabinoid system in pain-depression comorbidity. International Journal of Neuropsychopharmacology 19,pyv095. https://dx.doi.org/10.1093/ijnp/pyv095
Fusar-Poli L et al. (2020). Cannabinoids for people with ASD: A systematic review of published and ongoing studies. Brain Sciences 10(9), 572. https://dx.doi.org/10.3390/brainsci10090572
Häuser W et al. (2018) Efficacy, tolerability and safety of cannabis-based medicines for chronic pain management – An overview of systematic reviews. European Journal of Pain 22(3), 455-470. https://doi.org/10.1002/ejp.1118.
Henry RJ et al. (2016) For whom the endocannabinoid tolls: Modulation of innate immune function and implications for psychiatric disorders. Progress in Neuropsychopharmacology and Biological Psychiatry 64,167-180. https://dx.doi.org/10.1016/j.pnpbp.2015.03.006
Karhson DS et al. (2018). Plasma anandamide concentrations are lower in children with autism spectrum disorder. Molecular Autism 9,18 https://dx.doi.org/10.1186/s13229-018-0203-y
Kelly R et al. (2020). Microglial Phenotypes and Their Relationship to the Cannabinoid System: Therapeutic Implications for Parkinson’s Disease. Molecules 25 https://dx.doi.org/10.3390/molecules25030453
Kerr DM et al. (2016). Pharmacological inhibition of fatty acid amide hydrolase attenuates social behavioural deficits in male rats prenatally exposed to valproic acid. Pharmacological Research 113,228-235. https://dx.doi.org/10.1016/j.phrs.2016.08.033
Lowe H et al. (2021). The endocannabinoid system: a potential target for the treatment of various diseases. International Journal of Molecular Sciences 22(17). https://dx.doi.org/10.3390/ijms22179472
Silva EAdSJ et al. (2021) Cannabis and cannabinoid use in autism spectrum disorder: a systematic review. Trends in Psychiatry and Psychotherapy https://dx.doi.org/10.47626/2237-6089-2020-0149
Soliman N et al. (2021). Systematic review and meta- analysis of cannabis-based medicines, cannabinoids and endocannabinoid system modulators tested for antinociceptive effects in animal models of injury- related or pathological persistent pain. Pain https://dx.doi.org/10.1097/j.pain.0000000000002269
Thornton AM et al. (2021). Increasing endocannabinoid tone alters anxiety-like and stress coping behaviour in female rats prenatally exposed to valproic acid. Molecules 26(12). https://dx.doi.org/10.3390/molecules26123720
Wang J (2019). Glial endocannabinoid system in pain modulation. International Journal of Neuroscience 129, 94-100. https://dx.doi.org/10.1080/00207454.2018.1503178
Zamberletti E et al. (2017). The endocannabinoid system and autism spectrum disorders: insights from animal models. International Journal of Molecular Sciences 18 https://dx.doi.org/10.3390/ijms18091916