
Physiology News Magazine
Vitamin D
One hundred years of the sunshine vitamin
Features
Vitamin D
One hundred years of the sunshine vitamin
Features
https://doi.org/10.36866/pn.25.16
Dr Alexander T Carswell, University of East Anglia, Norwich, UK
Professor William D Fraser, University of East Anglia, Norwich, UK
A century ago, the industrialised towns and cities of Northern Europe and North America were filled with dark streets overshadowed by buildings, and covered by skies polluted by the smog of industry (Fig.1). Winter daylight hours were short and sunshine weak. Residents were exposed to limited sunlight and endured a post-war diet with little milk, cheese, butter, cooking fats, or meat. Rickets — a painful and disabling childhood disease characterised by weak, soft bones — was endemic with an estimated prevalence of 75–98%. The long bones in the legs of children with rickets were so poorly mineralised they could not resist the forces of tension and compression applied by their body’s weight. This caused lateral bending and a bow-legged or knock-knee appearance (see cover). Although rickets was first described in the 17th century, its cause remained unknown. Pioneering studies in the early 20th century showed that cod liver oil and sunlight could prevent and cure rickets. This led to the identification of an anti-rachitic factor crucial for bone mineralisation, named vitamin D, and commenced a century of fascinating research.

A, B, C, D — the discovery of the fourth vitamin
Following the understanding that diseases like beriberi and scurvy were caused by dietary deficiencies in vitamins B and C, respectively, it was thought that rickets might similarly be caused by a nutrient deficiency. At the start of the 20th century, rickets was so wildly prevalent in the UK that it was known as “the English disease”. In response to this and its possible dietary cause, the biochemist Edward Mellanby demonstrated in 1919 that dogs caged indoors (thereby inadvertently deprived of the sun’s ultraviolet radiation), and fed a diet of mainly oats, developed rickets. Mellanby found that cod liver oil was able to cure the disease. Vitamin A was thought by Mellanby to be the nutritional factor in cod liver oil responsible for its curative properties. Testing this hypothesis in 1922, Elmer McCollum and colleagues in the US cleverly found that destroying vitamin A within cod liver oil did not impair its anti-rachitic ability. They concluded that the nutritional factor responsible for curing rickets was a distinct vitamin, the fourth to be identified, and thus named it vitamin D (DeLuca, 2016).
In Austria, Kurt Huldschinsky in 1919, and Harriette Chick and colleagues in 1923, discovered that rickets could also be cured by exposing rachitic children to summer sunlight or artificial ultraviolet light. Similarly, the US-based laboratories of Harry Steenbock and Alfred Hess independently demonstrated in 1924 that irradiating animals or their food with ultraviolet light could prevent or cure rickets. With these apparently shared curative properties of cod liver oil and sunlight, Steenbock demonstrated in 1925 that irradiation converted an inactive lipid into the anti-rachitic factor (i.e. vitamin D) (DeLuca, 2016). These monumental discoveries led to the use of sun lamps to irradiate children, as well as the introduction of staple foods enriched with vitamin D, ultimately eliminating rickets as a major medical problem.

Vitamin D metabolism
In the 1930s, the structure of vitamin D2 was first identified by Askew et al. in the UK. Adolf Windaus and colleagues in Germany then isolated and identified the structure of vitamin D3 for the first time (DeLuca, 2016). Vitamin D2 is the plant-derived form, and vitamin D3 the endogenous form synthesised in the skin. Vitamin D2 was identified by irradiating plant sterols, and vitamin D3 by irradiating 7-dehydrocholesterol, its lipid precursor within the skin. Ultraviolet B radiation is absorbed by 7-dehydrocholesterol to form pre-vitamin D3 , which is converted to vitamin D3 , before entering the bloodstream bound to vitamin D binding protein (Fig.2). Vitamin D is fat soluble. Dietary vitamin D2 and D3 are incorporated into chylomicrons (large lipoprotein particles) before entering the lymphatic system and then the circulation also bound to vitamin D binding protein.
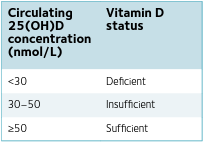
In the liver, vitamin D undergoes a hydroxylation reaction to form 25-hydroxyvitamin D [25(OH)D], the major circulating metabolite, the concentration of which is used to determine vitamin D status (Table 1). In the kidneys, 25(OH)D is hydroxylated to 1,25-dihydroxyvitamin D [1,25(OH)2 D], the biologically active form of vitamin D (Fig.2). The structures of 25(OH)D and 1,25(OH)2 D were identified in the US by Michael Holick, Hector DeLuca, and colleagues in the 1960s and 1970s.
To limit excessive 1,25(OH)2 D synthesis and activity, the renal hydroxylation reaction producing 1,25(OH)2 D is tightly regulated by parathyroid hormone, vitamin D itself, and phosphate. Parathyroid hormone stimulates hydroxylation, whereas 1,25(OH)2 D and phosphate inhibit 1,25(OH)2 D production. Although still categorised as a vitamin, the name “vitamin D” is a misnomer. Due to its endogenous synthesis in the skin, and owing to the fact that even the fraction gained from the diet (typically 10%) must be metabolised to its active form, vitamin D is actually a steroid hormone.
Bone mineralisation
The primary and classical physiological function of vitamin D is to maintain calcium and phosphate concentrations within their normal physiological ranges. Accordingly, vitamin D is essential for the mineralisation of bone. Serum 1,25(OH)2 D binds with nuclear vitamin D receptors (a transcription factor identified in 1973) to induce the expression of vitamin D target genes. The effects of this are three-fold: 1) augmentation of calcium absorption within the small intestine by increasing the expression of epithelial calcium channels and pumps; 2) promotion of renal calcium reabsorption in the distal tubule; 3) stimulation of osteoclastic bone resorption to release calcium into the circulation. Vitamin D also promotes phosphate absorption in the small intestine, reabsorption in the kidney, and resorption in bone. The 1952 discovery that vitamin D causes the mobilisation of calcium from bone into the circulation (i.e. decalcification) may appear counterproductive for bone strength. However, the indirect effects of vitamin D on bone (in the small intestine and kidney) supersede the direct effects. This results in a net increase in the flux of calcium into bone and augmented mineralisation of osteoid (Fig.2).
Beyond bone
The identification of vitamin D receptors in numerous body tissues indicates vitamin D has functions beyond maintaining calcium and phosphate homeostasis (Rosen et al., 2012). Evidence of extraskeletal effects of vitamin D has accumulated in the 21st century, including direct effects on aspects of innate and acquired immunity, skeletal and smooth muscle, and the cardiovascular system. Some effects of 1,25(OH)2 D are more immediate that those requiring gene transcription and translation and are understood to be mediated by membranebound vitamin D receptors and non-genomic pathways. Vitamin D has been shown to enhance the innate immune response by inducing antimicrobial proteins, which, in forming a first line of defence against invading pathogens, may help prevent upper respiratory tract infections (Martineau et al., 2017). Vitamin D may also have an anti-inflammatory or tolerogenic effect, whereby the severity or duration of infection symptoms are reduced. Despite the noise from some underpowered trials and unreliable observational studies at the height of the pandemic, evidence is sparse for a consensus to be reached on whether vitamin D can reduce the severity of COVID-19. Vitamin D may have a role in protecting against cancer and reducing cancer development by enhancing cell apoptosis and inhibiting cell proliferation; however, the strength of the existing evidence is insufficient for a definitive conclusion to be reached (Scientific Advisory Committee on Nutrition, 2016). The development, repair, and remodelling of skeletal muscle may be influenced by vitamin D, giving it the ability to affect muscle function. Vitamin D has been demonstrated to affect muscle metabolism and intracellular calcium handling, and to improve mitochondrial oxidative function (Girgis et al., 2014). Vitamin D has also been shown to mitigate endothelial dysfunction (potentially by stimulating the release of the vasodilator nitric oxide) and may, therefore, be beneficial for cardiovascular health (Wolf et al., 2020).
The next one hundred years…?
Cause and effect
Controversy about the extent of vitamin D’s influence beyond bone mineralisation exists. With most evidence derived from correlative observational studies, there is a paucity of research demonstrating cause and effect. Positive associations between vitamin D status and physical performance or cardiovascular health may be due to reverse causation; whereby more active, healthier individuals may spend more time outdoors exposed to sunlight, and thereby have superior vitamin D status than more sedentary individuals. Two recent, large, randomised, double-blind, placebo-controlled trials found that vitamin D supplementation did not reduce the incidence of cancer, cardiovascular events (Manson et al., 2019), or type 2 diabetes (Pittas et al., 2019). Further empirical data are needed to clearly elucidate the role of vitamin D metabolites on extraskeletal outcomes, whilst controlling for baseline 25(OH)D concentrations. For beneficial or ergogenic effects of vitamin D to be detected, vitamin D-deficient individuals ought to be targeted (Table 1). However, this approach will raise ethical concerns for individuals allocated to control arms.
Endemic deficiency
Even in the 21st century, vitamin D deficiency and rickets continue to be a problem in some regions (e.g. North Africa and the Middle East) and ethnic groups (e.g. South Asians and Africans living in high-income countries), particularly where exposure to sunlight is compromised, for instance by residing indoors during daylight hours or wearing clothing that covers the skin. Individuals with darker skin (containing more melanin) who have emigrated to temperate latitudes are at increased risk of deficiency due to melanin’s absorption of ultraviolet radiation. Recommended safe sunlight exposure guidelines for vitamin D sufficiency (15 min daily UK summer sunlight exposure to one third of the skin surface area [Advisory Group on Non-ionising Radiation, 2017]) need to be updated to include advice for non-white skinned individuals.
Recommended intake
Debate continues around the thresholds for vitamin D deficiency, insufficiency, sufficiency, and a proposed optimum vitamin D status. Defined for skeletal health (Table 1), evidence is lacking to support specific recommendations for cardiovascular health, physical performance, or protection against respiratory infections. Consequently, recommended daily intakes of vitamin D continue to be debated. In the UK, a vitamin D reference nutrient intake/ safe intake of 400 IU/day is recommended for everyone aged ≥1 year to maintain serum 25(OH)D ≥25 nmol/L (Scientific Advisory Committee on Nutrition, 2016). At UK latitudes (50–60°N), the strength of solar ultraviolet B radiation is inadequate to make enough vitamin D between October and March, therefore the UK government recommends everyone should consider taking a daily supplement containing 400 IU vitamin D3 during these months. An upper intake limit of 4000 IU/ day is advised to avoid vitamin D intoxication and hypercalcaemia. Even without evidence of toxicity, high-dose vitamin D supplementation may be detrimental (Owens et al., 2017).
Metabolites
Serum 25(OH)D has recently been purported to be a poor biomarker of vitamin D status, despite its use for decades. Serum 25(OH)D concentration does not differentiate between 25(OH)D bound to vitamin D binding protein or albumin (approximately 99%), and unbound (and thus bioavailable) 25(OH)D. Bioavailable metabolites may more strongly correlate with clinical outcomes. The ratio of 24,25-dihydroxyvitamin D [24,25(OH)2 D, a product of 25(OH)D] to 25(OH)D is independent of vitamin D binding protein concentrations and might be a better marker of vitamin D status (Ginsberg et al., 2021).
Most vitamin D metabolites (33 have been identified) are deemed to be inactive or intermediary waste products. However, thanks to recent advances in technology enabling the separation and quantification of metabolites, interest in possible biological actions and variation in their relative concentrations has risen (Tang et al., 2019). Potential functions of 24,25(OH)2 D in fracture repair, and the existence of a 24,25(OH)2 D-specific receptor have emerged. Whether supplementation with metabolites downstream of 25(OH)D can positively influence clinical or other meaningful outcomes warrants investigation.
Genetics
Polymorphisms in the genes that encode for the vitamin D receptor, binding protein, or hydroxylation enzymes may be responsible for some inter-individual variability in the vitamin D endocrine system (Girgis et al., 2014). The impact of an individual’s genotype on vitamin D status, and physiological function remains to be fully determined.
Ageing
Lower availability of 7-dehydrocholesterol in ageing skin and reduced sun exposure in the frail are risk factors for vitamin D deficiency. Vitamin D-deficient adults can develop osteomalacia, which is characterised by weak, inadequately mineralised bones at increased risk of fracture. Vitamin D deficiency during adulthood can also contribute to and exacerbate osteopenia (loss of bone mass) and osteoporosis, heightening fracture risk. With life expectancies increasing globally, and the ageing demographics of developed countries, maintaining vitamin D sufficiency from cradle to grave is of increasing importance, at least to maximise the accumulation and maintenance of bone mineral density. Biofortification of a wider array of foods with vitamin D may be a convenient and cost-effective means of providing an adequate vitamin D intake throughout the lifecycle. Vitamin D is stored in body fat. With the prevalence of overweight and obese individuals increasing, releasing vitamin D from adipose tissue could prove to be an effective way to avoid vitamin D deficiency.
Climate change
More frequent extremes of low and high temperatures due to global warming may result in reduced skin exposure to sunlight (with individuals covering their skin with clothing or sunscreen, and spending more time indoors). Rising temperatures may also accelerate migration away from the equator to more temperate latitudes. Climate change could therefore reduce endogenous vitamin D synthesis and increase the risk of vitamin D deficiency. Appropriate supplementation and safe sun exposure guidance to mitigate these knock-on effects of climate change may be necessary.
Final thought
If the 1920s were the dawn for the study of vitamin D, the sun is far from setting on this remarkable molecule.
References
Advisory Group on Non-Ionising Radiation (2017). Ultraviolet radiation, vitamin D and health. London: Public Health England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/620184/UV_Radiation__Vitamin_D___Health.pdf.
Deluca HF (2016). Vitamin D: historical overview. Vitamins and Hormones 100, 1-20. http://doi.org/10.1016/bs.vh.2015.11.001.
Ginsberg C et al. (2021). The vitamin D metabolite ratio is associated with changes in bone density and fracture risk in older adults. Journal of Bone and Mineral Research 36(12), 2343-2350. http://doi.org./10.1002/jbmr.4426.
Girgis CM et al. (2014). Effects of vitamin D in skeletal muscle: falls, strength, athletic performance and insulin sensitivity. Clinical Endocrinology (Oxford) 80(2), 169-181. http://doi.org/10.1111/cen.12368.
Manson JE et al. (2019). Vitamin D supplements and prevention of cancer and cardiovascular disease. The New England Journal of Medicine 380(1), 33-44. http://doi.org/10.1056/NEJMoa1809944.
Martineau AR et al. (2017). Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 356, i6583. http://doi.org/10.1136/bmj.i6583.
Owens DJ et al. (2017). Efficacy of high-dose vitamin D supplements for elite athletes. Medicine & Science in Sports & Exercise 49(2), 349-356. http://doi.org/10.1249/MSS.0000000000001105.
Pittas AG et al. (2019). Vitamin D supplementation and prevention of type 2 diabetes. The New England Journal of Medicine 381(6), 520-530. http://doi.org/10.1056/NEJMoa1900906.
Rosen CJ et al. (2012). The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocrine Reviews 33(3), 456-492. http://doi.org/10.1210/er.2012-1000.
Scientific Advisory Committee on Nutrition (2016). Vitamin D and health. London: Public Health England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/537616/SACN_Vitamin_D_and_Health_report.pdf.
Tang JCY et al. (2019). The dynamic relationships between the active and catabolic vitamin D metabolites, their ratios, and associations with PTH. Scientific Reports 9(1), 6974. http://doi.org/10.1038/s41598-019-43462-6.
Wolf ST et al. (2020). Four weeks of vitamin D supplementation improves nitric oxide-mediated microvascular function in college-aged African Americans. American Journal of Physiology Heart and Circulatory Physiology 319(4), H906-H914. http://doi.org/10.1152/ajpheart.00631.2020.
