
Physiology News Magazine
Stem cell-based therapies for musculoskeletal regeneration
Role of the microenvironment in augmenting regenerative medicine therapies by elucidating stem cell physiology
Features
Stem cell-based therapies for musculoskeletal regeneration
Role of the microenvironment in augmenting regenerative medicine therapies by elucidating stem cell physiology
Features
https://doi.org/10.36866/pn.25.24

Dr Rahul S Tare
University of Southampton, UK
All the information required to define a multicellular organism resides in a single cell, the zygote or the single-cell embryo. And therein lies the origins of the concept of a “stem cell” as a highly specialised cell containing all the information required to generate a complex multicellular organism. Stem cells are a key component of the multidisciplinary tissue engineering paradigm used for the generation of living tissues and organs ex vivo (outside the body). The bioengineered tissues and organs are implanted in vivo to improve or restore normal biological function in regenerative medicine therapies for disorders of complex organ systems such as the musculoskeletal system. A wide array of regenerative medicine strategies, ranging from stem cell-based therapies to the application of tissue-engineered products, have been applied for the treatment of bone defects, articular cartilage lesions, disorders of the spine and tendon/ligament injuries. Further advances in the development of improved musculoskeletal regenerative medicine therapies will be guided by a detailed understanding of underlying mechanisms governing the homeostasis between stem cell renewal and differentiation.
Stem cells reside in a specialised microenvironment, the stem cell niche, and differentiate in diverse tissue microenvironments after circulating away from their niches. Dissecting the key roles of the stem cell niche and the diverse tissue microenvironments in regulating stem cell self-renewal and lineage commitment will contribute to greater understanding of the underlying mechanisms governing stem cell function. Additionally, comprehensive insight into the interactions between stem cells and other components of the stem cell niche, and the effect of extracellular matrix (ECM) elasticity/stiffness on stem cell lineage specification in diverse tissue microenvironments will inform strategies for improved stem cell culture and differentiation into desired musculoskeletal lineages.

Types and characteristics of stem cells used in musculoskeletal regeneration
Stem cells are defined as highly specialised cells characterised by the capacity for prolonged self-renewal under controlled conditions and, while maintaining their undifferentiated state, are able to differentiate into a variety of mature cell types based on their varying differentiation potential or potency. Based on their differentiation ability, stem cells are categorised as:
- totipotent – able to differentiate into all cell types and extra-embryonic tissues (e.g. the zygote)
- pluripotent – able to differentiate into all cell types of the three germ layers e.g. embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs))
- multipotent – able to differentiate into the multiple cell types constituting the tissue of origin (e.g. haematopoietic stem cells, mesenchymal stem cells (MSCs))
- unipotent – able to differentiate into a single cell type (e.g. spermatogonia giving rise only to the sperm)
ESCs constitute an inexhaustible source of self-renewing cells capable of differentiating into the principal musculoskeletal lineages, namely bone, cartilage, muscle and fat, making them a viable option for application in musculoskeletal regeneration. Allogeneic human ESCs (hESCs) have been successfully differentiated into chondrocytes, which in turn have been used to tissue engineer mechanically competent 3D hyaline cartilage constructs that have the potential to repair focal defects in articular cartilage, widely regarded to be an immune-privileged skeletal tissue (Griffith et al., 2021). However, ethical concerns related to the embryonic origin of hESCs, predisposition of hESCs to form teratomas and the risk of immune rejection due to debatable immune-privileged properties have limited the application of hESCs in musculoskeletal regeneration. While potential issues with immunogenicity could be ameliorated by generating banks of clinical-grade hESC lines, which are human leukocyte antigenmatched to groups of individuals, it is important that the additional challenge of aneuploidy (presence of an abnormal number of chromosomes) arising from the prolonged culture of hESCs is addressed before the application of hESCs in the clinic.
Human iPSCs (hiPSCs) circumvent the ethical issues associated with the use of hESCs and constitute an autologous pluripotent stem cell population suited for use in personalised regenerative medicine approaches. However, there are challenges related to their use. These include biased differentiation potential of hiPSCs into their lineage of origin due to the retention of residual epigenetic memory from the donor cell source, potential tumorigenicity, genetic instability and phenotypic heterogeneity. Derivation of clinical-grade hiPSCs using transient, integration-free methods for the delivery of reprogramming factors without causing insertional inactivation of tumour suppressor genes and/or activation of oncogenes also remains challenging.
The term “mesenchymal stem cell” was originally coined to describe a hypothetical common progenitor of a wide range of nonhaematopoietic, non-epithelial, mesodermal tissues. MSCs used in musculoskeletal regeneration have been isolated from umbilical cord blood and an array of adult tissues. These include postnatal human bone marrow stromal tissue (referred to as skeletal stem cells (SSCs)), adipose tissue, skeletal muscles (referred to as satellite cells), cartilage, synovium, ligaments and tendons. Multipotent MSCs are acknowledged as promising autologous cell populations for musculoskeletal regeneration due to their relative ease of isolation and expansion in vitro, perceived immunomodulatory properties, limited tumorigenicity and robust ability to differentiate into the major cell types of the musculoskeletal system due to their mesodermal origin.
The application of MSCs for musculoskeletal regeneration requires expansion of the MSC populations under defined conditions to generate an optimal number of cells without altering their phenotype or genotype. However, bone marrow-derived MSCs (BMSCs), the most widely used MSC population in musculoskeletal regeneration, exhibit limited cell proliferation ability and “replicative senescence” following successive subculture (Stenderup et al., 2003). Furthermore, the ability of BMSCs to proliferate and differentiate declines with advancing age and they demonstrate considerable heterogeneity in their growth rate and differentiation potential (Phinney et al., 1999; Stenderup et al., 2003). Although there are no reports of spontaneous transformation of culture-expanded BMSCs and culture-induced genetic alterations in the BMSCs that could lead to tumour formation in vivo, it has been suggested that systemically administered BMSCs could be recruited to the tumour stroma and promote the growth of a latent tumour, as evidenced in some experimental cancer models (Lepperdinger et al., 2008).
The overall safety record of BMSCs remains excellent; their ready accessibility from bone marrow and ability to differentiate into the principal skeletal cell lineages in vivo have driven the clinical application of this autologous MSC population in patienttailored therapies for musculoskeletal regeneration.
MSC-based therapies for musculoskeletal regeneration
The surgical technique of microfracture used to treat defects in articular cartilage relies on the creation of tiny fractures in the bone underlying the articular cartilage to stimulate the influx of SSCs and growth factors from the bone marrow to promote defect repair. MSC-enriched bone marrow, or concentrates thereof, or in vitro cultured MSCs have been directly injected into diseased tissue, such as the osteoarthritic knee joint, where the cells eventually populate the target site and stimulate repair via autocrine or paracrine pathways (Davatchi et al., 2011; Emadedin et al., 2012; Wong et al., 2013). Similarly, injections of adipose tissue-derived MSCs (ADMSCs) are used to treat tendonitis as they are capable of differentiating into tendons.
When large defects in bone (caused by trauma, tumours, infection, aseptic loosening, or non-unions) and cartilage (caused by daily wear and tear, trauma due to sports injuries etc.) need to be repaired, stem cell delivery to the defect site is augmented by using biomaterials. Examples include the delivery of autologous MSCs using osteoconductive hydroxyapatite and calcium phosphate scaffolds to successfully treat large segmental bone defects (Quarto et al., 2001). Osteocel® Plus, an advanced allograft cellular bone matrix containing MSCs and osteoprogenitor cells combined with demineralised bone matrix and cancellous bone, and the Trinity EvolutionTM allograft, comprising cancellous bone with viable osteogenic and osteoprogenitor cells retained within the matrix and a demineralised cortical bone component, have been used as substitutes for conventional autografts/allografts in spinal fusion and foot/ankle fusion surgeries (Rush, 2010; Ammerman et al., 2013).
A mechanically stable living bone composite comprising of SSCs and milled allograft was impacted into necrotic bone in the femoral head and was shown to be an effective new treatment for focal early-stage avascular necrosis of the femoral head (Aarvold et al., Surgeon, 2013). Cartistem®, a medicinal product comprising of culture-expanded allogeneic human umbilical cord blood-derived MSCs delivered using the hyaluronic acid hydrogel, was applied for the regeneration of painful full-thickness cartilage defects in patients with osteoarthritis of the knee joint (Park et al., 2017).
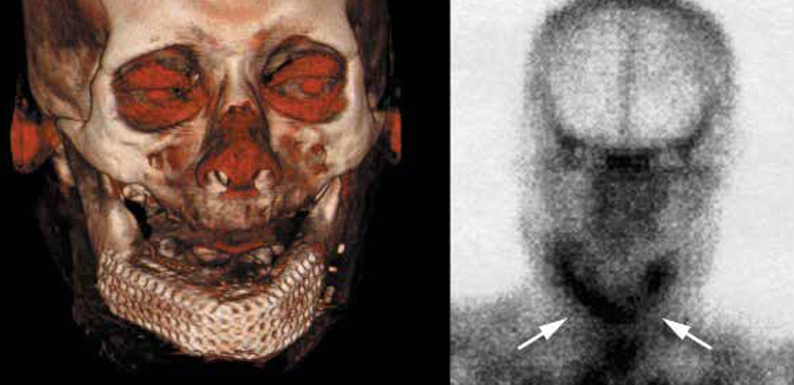
Biomaterial-assisted cell delivery is further enhanced by the application of chemical cues, provided by growth factors and supplements, and mechanical stimuli, via dynamic culture in bioreactors, which facilitate the generation of 3D tissue-engineered constructs ex vivo. Upon implantation in vivo, tissue-engineered constructs have the potential to fill the entirety of the defects, integrate effectively with the surrounding host tissue, being structurally and mechanically analogous to the host tissue, and promote robust repair. Advances in tissue engineering have included the application of 3D computed tomography (CT) scanning and computer-aided design to bioengineer a bone graft, which was perfectly shaped in the form of the patient’s lower jaw bone/mandible, by fabricating a titanium mesh cage that was filled with hydroxyapatite, infiltrated with recombinant human bone morphogenetic protein (osteogenic growth factor) and the patient’s bone marrow containing SSCs (Fig.2). Following implantation of the vascularised bone graft into the lower jaw, the patient had an improved degree of mastication and was satisfied with the aesthetic outcome of the procedure (Warnke et al., 2004). Similarly, bespoke titanium joint implants, fabricated using computer-aided design-computer-assisted manufacturing, were enhanced with autologous SSCs and used to treat patients with significant bone loss due to failed joint replacements, resulting in significant clinical and radiological improvements (Goriainov et al., 2018).
Although significant advances have been made in stem cell-based musculoskeletal regenerative therapies, especially for bone and cartilage disorders, further developments in this field will be guided by comprehensive understanding of stem cell physiology and, critically, how the microenvironment modulates stem cell function in response to physiological challenges.
Role of the microenvironment in stem cell self-renewal and lineage specification
Stem cells reside in the stem cell niche, a specialised microenvironment constituting the basic unit of tissue physiology. The niche prevents depletion of the stem cell pool, protects the host from excessive stem cell proliferation and integrates the signals that orchestrate stem cell lineage specification and participation in tissue generation, maintenance and repair. The niche is composed of supporting cells located in unique topological relationships with the stem cells, signalling factors and the ECM. Biochemical signals from the supporting cells in the stem cell niche have been identified as important paracrine regulators of stem cell function. Moreover, the ECM is a multifaceted component of the niche as, in addition to its structural role, it regulates stem cell function by integrating the mechanical and biochemical signals through mechanotransduction – the process of translating mechanical forces through signalling cascades to affect changes in cells.
The niche also functions as a physical anchor for stem cells, with adhesion molecules such as integrins anchoring the stem cells to the ECM. The stem cell retains its ability to self-renew by maintaining close contact with the niche. Occasionally, the stem cell divides parallel to the niche surface, ensuring that both daughter cells maintain contact with the niche and retain the ability to self-renew, thereby generating two stem cells. In contrast, by dividing perpendicular to the niche surface, the stem cell ensures that only one of the two daughter cells maintains contact with the niche and retains the ability to self-renew, while the other daughter cell leaves the stem cell niche to differentiate into a functionally mature cell. Thus, niche-controlled stem cell divisions offer a greater degree of flexibility and are more commonly observed in adult MSCs (Knoblich, 2008).
As adult stem cells circulate away from their niches and engraft and differentiate within a range of tissues, they are confronted with a range of ECM microenvironments as physically distinct as muscle, bone etc. MSCs exhibit extreme sensitivity to the elasticity/ stiffness of the ECM; matrix stiffness in turn directs MSC differentiation and commitment to a specific lineage or phenotype. A stiffer substrate mimicking the muscle matrix has been shown to drive MSC differentiation into the myogenic lineage, while a rigid matrix mimicking collagenous bone directs differentiation into an osteogenic lineage. Cytoskeletal nonmuscle myosin isoforms (A, B, C) are involved in sensing matrix elasticity and tensioning the cortical actin structures, which in turn are linked to the focal-adhesion complexes that activate signalling molecules, the mechanotransducers, within the stem cells to affect phenotypic change (Engler et al., 2006).
Thus, comprehensive understanding of the complex interplay between stem cells and their niches that regulates stem cell function, and the effect of the ECM associated with the diverse musculoskeletal microenvironments on stem cell lineage commitment, will inform the design of successful stem cellbased regenerative medicine strategies for musculoskeletal repair.
Future directions
A thorough understanding of the constituents of the stem cell niche and dissection of the complex interactions between stem cells and other components of the niche provide a unique opportunity to reconstitute the stem cell niche in vitro, facilitating the successful expansion of stem cells and generation of reservoirs of stem cells for use in musculoskeletal regeneration therapies. Furthermore, greater insight into matrix elasticity-directed MSC lineage specification opens up avenues to design and fabricate customised biomaterials with optimum elastic properties that promote MSC differentiation into desired lineages to foster robust musculoskeletal regeneration.
References
Aarvold A et al. (2013). A tissue engineering strategy for the treatment of avascular necrosis of the femoral head. The Surgeon: Journal of the Royal College of Surgeons of Edinburgh and Ireland 11, 319-325. https://doi. org/10.1016/j.surge.2013.02.008.
Ammerman JM et al. (2013). The role of Osteocel Plus as a fusion substrate in minimally invasive instrumented transforaminal lumbar interbody fusion. Clinical Neurology and Neurosurgery 115, 991-994. https:// doi.org/10.1016/j.clineuro.2012.10.013.
Davatchi F et al. (2011). Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. International Journal of Rheumatic Diseases 14, 211-215. https://doi.org/10.1111/j.1756185X.2011.01599.x.
Emadedin M et al. (2012). Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Archives of Iranian Medicine 15, 422-428. PMID: 22724879.
Engler AJ et al. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677-689. https://doi. org/10.1016/j.cell.2006.06.044.
Goriainov V et al. (2018). Application of 3D-printed patient-specific skeletal implants augmented with autologous skeletal stem cells. Regenerative Medicine 13, 283-294. https://doi.org/10.2217/rme-20170127.
Griffith LA et al. (2021). A scaffold-free approach to cartilage tissue generation using human embryonic stem cells. Scientific Reports 11, 18921. https://doi. org/10.1038/s41598-021-97934-9.
Knoblich JA (2008). Mechanisms of asymmetric stem cell division. Cell 132, 583-597. https://doi. org/10.1016/j.cell.2008.02.007.
Lepperdinger G et al. (2008). Controversial issue: Is it safe to employ mesenchymal stem cells in cell-based therapies? Experimental Gerontology 43, 1018–1023. https://doi.org/10.1016/j.exger.2008.07.004.
Park YB et al. (2017). Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Translational Medicine 6, 613621. https://doi.org/10.5966/sctm.2016-0157.
Phinney DG et al. (1999). Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. Journal of Cellular Biochemistry 75, 424–436. PMID: 10536366.
Quarto R et al. (2001). Repair of large bone defects with the use of autologous bone marrow stromal cells. The New England Journal of Medicine 344, 385-386. https://doi.org/10.1056/NEJM200102013440516.
Rush SM (2010). Trinity Evolution: mesenchymal stem cell allografting in foot and ankle surgery. Foot & Ankle Specialist 3, 140-143. https://doi. org/10.1177/1938640010369638.
Stenderup K et al. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33, 919–926. https://doi.org/10.1016/j.bone.2003.07.005.
Warnke PH et al. (2004). Growth and transplantation of a custom vascularised bone graft in a man. Lancet 364, 766-770. https://doi.org/10.1016/S01406736(04)16935-3.
Wong KL et al. (2013). Injectable cultured bone marrowderived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 29, 2020-2028. https:// doi.org/10.1016/j.arthro.2013.09.074.
