
Physiology News Magazine
Phototransduction
The decline and fall of the calcium theory
Features
Phototransduction
The decline and fall of the calcium theory
Features
https://doi.org/10.36866/pn.25.20
Dr Jonathan A. Coles, Honorary Research Fellow, University of Glasgow
The death of the calcium theory of phototransduction was announced after lunch at the Zuricher Hotel in Berlin on Friday 30 November 1984 to an international audience of some 40 researchers working on vision. The announcement instantly resolved a fierce debate that had been going on for almost 20 years and we were given the afternoon off to try to recover from the shock.
In 1965 when the pathway of sensory transduction from stimulus to electrical signal was unknown for any of the senses, vision had the attraction of the precision of the stimulus: a photon absorbed by a rhodopsin molecule. It was known that most signalling in the nervous system resulted from an increase in membrane permeability, particularly to sodium, whose influx depolarised the cell. Some invertebrate photoreceptors, such as the giant cells of the ventral eye of the horseshoe crab, Limulus, had been studied and they depolarised in response to light, as expected. In vertebrate retina, Svaetichin (1953) recorded from sites with a negative resting potential that gave a hyperpolarising, rather than a depolarising, response to light. Svaetichin’s claim that these recordings were from photoreceptors was met with justifiable scepticism – for one thing, these recordings were too easy to make. But it took several groups, working for over a decade, to understand the situation (see Tomita, 1965 for refs). Oikawa et al. (1959), working on carp retinas, showed that in most recordings, the response amplitude increased when the area stimulated by light increased, but in some it did not. They suggested that the first class were horizontal cells (which extend over an area including many photoreceptors) and the second class were the photoreceptors themselves, in this case, cones. Bortoff (1964) recorded from cells in the salamander, Necturus, which he identified by dye labelling as rod photoreceptors and again these gave hyperpolarising responses to light. Meanwhile, Tsuneo Tomita and his students in Tokyo were working carefully and methodically to decipher the system. They introduced a range of new techniques, most notably a jolter that used an electromagnetic diaphragm to jolt the retina onto the microelectrode (Tomita et al., 1967; Kaneko, 2021). They also built an elaborate light stimulator that could measure the spectral response of a cell quickly, before the electrode fell out, and used it to show that responses from cones in carp supported Young’s trichromatic theory of colour vision. Toyoda et al. (1969) showed that the light-induced hyperpolarisation in rods was due to a fall in membrane conductance. This result defined a major problem: in rods, the rhodopsin that is isomerised by photons and initiates the receptor potential is in the membranes of the discs or sacs that are stacked inside the outer segment, and not attached to the surface membrane (Fig.1A).
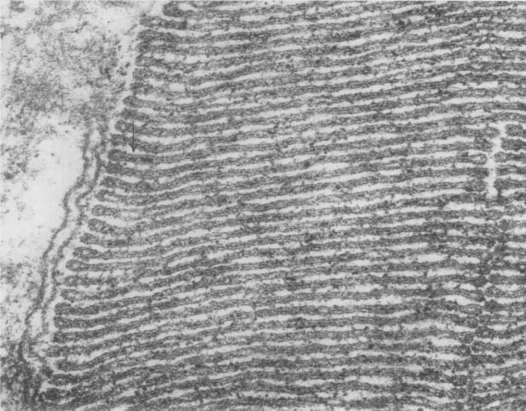
A tempting idea was that the photoisomerisation of rhodopsin caused some ion or molecule to diffuse through the cytoplasm from the disc and close channels on the surface membrane. Protons were quickly ruled out, but the hypothesis of Yoshikami and Hagins (1971) that the internal transmitter was calcium, received experimental support for a decade or so. On a different tack, cyclic adenosine monophosphate was known to be a common internal messenger and the enzymes required for its production (cyclase) and hydrolysis (phosphodiesterase) were looked for, and found, in photoreceptors. But in rod outer segments, it was the guanosine system, rather than the adenosine, that was far more active, both a guanylate cyclase (Goridis et al., 1973) and a guanylate phosphodiesterase. Photoisomerisation of a single rhodopsin molecule rapidly activated up to 500 molecules of phosphodiesterase converting cGMP to 5’GMP (Yee and Liebman, 1978). Manipulating cGMP levels changed the membrane potential. But how would a change in the concentration of cGMP change the membrane resistance? Its expected action was to phosphorylate proteins, or perhaps a light-activated GTPase was involved (Fleischman and Denisevich, 1979).
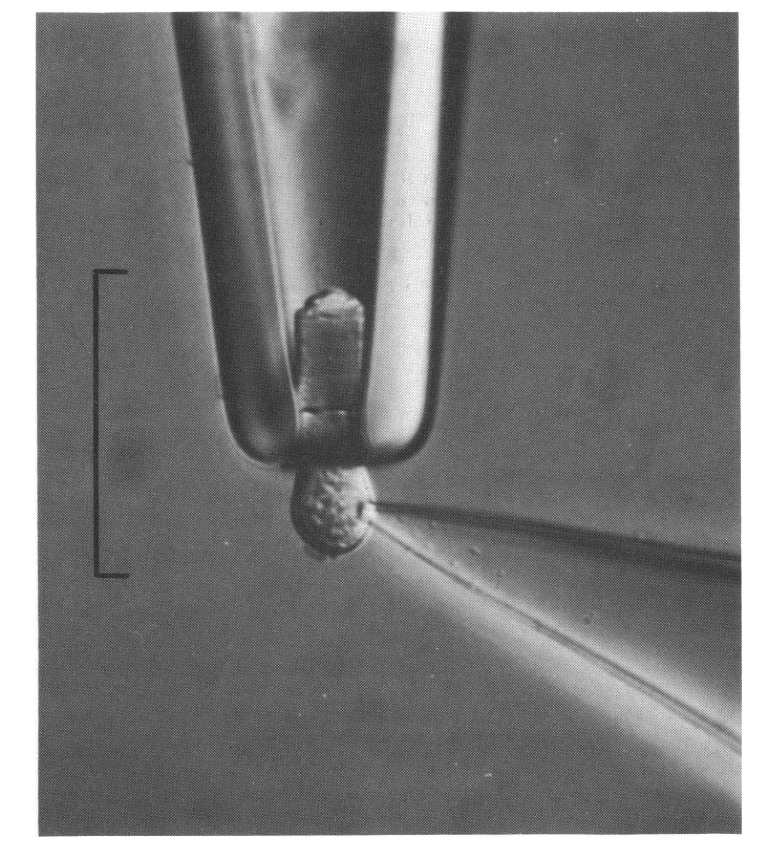
In the 1970s several new techniques were brought to bear. Yau and colleagues independently developed a way of measuring the current flowing through the rod outer segment membrane by sucking the outer segment into the tip of a pipette (Yau et al., 1977) and, with dexterity, this could be combined with the patch electrode technique definitively described by Hamill et al. (1981) (see Fig.1B). In 1980, Gold and Korenbrot devised a calcium-sensitive membrane electrode on which they placed a retina and showed that light stimulation caused calcium release from rod outer segments.
It should be remembered that news was transmitted by post, by visits and sometimes by telephone, and that checking the latest literature meant going to the library and thumbing through journals. Working out a coherent description of transduction in a vertebrate sensory cell was an obvious prize and papers came tumbling out, often beautifully done and with considered but inconclusive discussions; there were, for example, six papers in 1984 on the subject in Nature alone.
This was the background to the Dahlem “Konferenz” (more a “workshop”) that was held in 1984 on “The Molecular Mechanisms of Photoreception”. Dahlem, a suburb of Berlin, is where Otto Warburg had done his biochemistry research and, in 1933, it had a fleeting connection with phototransduction when George Wald visited and found vitamin A (retinal) in the retina. Since 1974, several Dahlem Workshops a year have been held in what was the enclave of West Berlin. They are limited to 40 participants, discussion documents are prepared beforehand and, for much of the time, the meeting is split into four working groups that produce reports on different aspects. For the 1984 phototransduction meeting, the scientific organiser was Hennig Stieve, who worked on Limulus ventral photoreceptor, and the detailed management was by Silke Bernhard, who had 10 years of experience. The dates were 25–30 November 1984, which turned out to be important. Among other things, a key paper on “Electrogenic Na-Ca exchange in retinal rod outer segment” by Yau and Nakatani (1984) had been published on 18 October.
I was something of an outsider and was shamefully relaxed about it. I enjoyed the luxury hotel overlooking the Tiergarten park, and the delightful small restaurants that Silke, a perfect host, took us to in the evenings. Of course, all this was in the Western sector. Despite the Berlin Wall, the underground railway could be used to cross the border, the main incentive being to visit the Pergamon museum with its outstanding collection of archeological treasures.
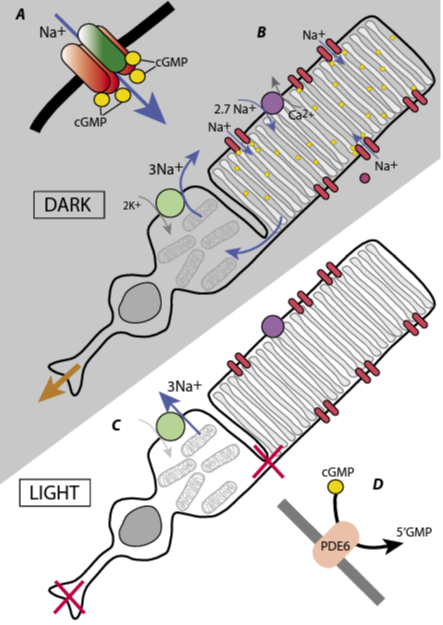
A rule at the Dahlem Workshops was that no slides were to be shown. However, early on in the meeting, Trevor Lamb, a tall, bearded New Zealander, twisted Silke’s arm and was allowed to show one slide, which showed that when intracellular calcium was chelated the photoresponse increased rather than decreased (Matthews et al., 1985). The meeting went on, with a curious air of uneasiness as nearly all the participants wondered what the role of cGMP was. Then, after lunch on the last day, it was revealed that two reviewers and a Nature Editor (all present at the meeting) had a manuscript received from the USSR Academy of Sciences at Pushchino, near Moscow, on 16 November, nine days before the meeting. Defying general wisdom, Fesenko, Kolesnikov and Lyubarsky (Fesenko et al., 1985) had applied cGMP directly to the cytoplasmic face of the surface membrane of a rod outer segment and found that it increased the conductance. This result filled the missing link in vertebrate phototransduction: photoisomerisation of rhodopsin led to activation of the phosphodiesterase, which reduced cGMP levels and decreased the membrane conductance (Fig.2). As the meeting report in Nature (Altman, 1985) put it, the announcement “electrified the whole meeting”. Disclosing results from a paper under review was not quite proper, and Nature had politely waited until publication of the Fesenko paper, on 24 January 1985, before publishing its report. Curiously, both this report, and the introduction to the proceedings book (Stieve, 1986) make the same mistake in the references, putting the publication of the Fesenko paper in 1984 rather than 1985, a subconscious attempt to smooth out history, perhaps. The Nature report also announced three further papers in press, all supporting the cGMP theory and these were published together on 14 February (Yau and Nakatani, 1985; Matthews et al., 1985; Cobbs and Pugh, 1985). The “received on” dates show that all three were under review at the time of the meeting, at which four of the authors were present (and, presumably, some of the reviewers). The atmosphere in the “Internal Messengers” working group must have been interesting.
What has happened since the meeting? Most of the participants continued long and productive careers in vision: a detailed understanding of the regulation of transduction by calcium, the molecular structures of the proteins and how those structures transform, the evolution of photoreceptors, the causes of eye disease. Of the three authors of the crucial paper, Kolesnikov and Lyubarsky continued on vision, Lyubarsky having moved to Philadelphia, while Kolesnikov stayed in Pushchino. Fesenko, also in Pushchino, continues to publish frenetically on subjects that usually include magnetic fields, electromagnetic radiation or cryoprotection.
References
Altman J (1985). Sensory transduction: new visions in photoreception. Nature 313(6000), 264-265. http://doi.org/10.1038/313264a0.
Bortoff A (1964). Localization of slow potential responses in the Necturus retina. Vision Research 4(11), 627-635. http://doi.org/10.1016/00426989(64)90048-3.
Cobbs JW, Pugh EDJ (1985). Cyclic GMP can increase rod outer-segment light-sensitive current 10-fold without delay of excitation. Nature 313(6003), 585587. http://doi.org/10.1038/313585a0.
Fesenko EE et al. (1985). Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature 313(6000), 310-313. http://doi.org/10.1038/313310a0.
Fleischman D, Denisevich M (1979). Guanylate cyclase of isolated bovine retinal rod axonemes. Biochemistry 18(23), 5060-5066. http://doi.org/10.1021/bi00590a006.
Gold GH, Korenbrot JI (1980). Light-induced calcium release by intact retinal rods. Proceedings of the National Academy of Sciences USA 77(9), 5557-5561. http://doi.org/10.1073/pnas.77.9.5557.
Goridis C et al. (1973). Guanyl cyclase in a mammalian photoreceptor. FEBS Letters 30(2), 163-166. http://doi.org/10.1016/0014-5793(73)80642-8.
Hamill OP et al. (1981). Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archives 391(2), 85-100. http://doi.org/10.1007/BF00656997.
Kaneko A (2021). Recollection of my research work on the electrophyiology of the vertebrate retina. Bioelectricity 3(3), 221-228. http://doi.org/10.1089/bioe.2021.0022.
Lamb TD et al. (1986). Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. Journal of Physiology 372(1), 315-349. http://doi.org/10.1113/jphysiol.1986.sp016011.
Matthews HR et al. (1985). Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods. Nature 313(6003), 582-585. http://doi.org/10.1038/313582a0.
Nilsson SE (1965). The ultrastructure of the receptor outer segments in the retina of the leopard frog (Rana Pipiens). Journal of Ultrastructure Research 12, 207-231. http://doi.org/10.1016/s00225320(65)80016-8.
Oikawa T et al. (1959) Origin of so-called cone action potential. Journal of Neurophysiology 22(1), 102-111. http://doi.org/10.1152/jn.1959.22.1.102.
Stieve S (ed.) 1986. The Molecular Mechanism of Photoreception, Berlin: Springer Verlag.
Svaetichin G (1953). The cone action potential. Acta Physiologica Scandinavica 29(Suppl 106), 565-600.
Tomita T (1965). Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harbor Symposia of Quantitative Biology 30, 559-566. http://doi.org/10.1101/sqb.1965.030.01.054.
Tomita T et al. (1967). Spectral response curves of single cones in the carp. Vision Research, 7(7), 519531. http://doi.org/10.1016/0042-6989(67)900612.
Toyoda J et al. (1969). Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Research 9(4), 453-463. http://doi.org/10.1016/0042-6989(69)90134-5.
Xue H et al. (2021). Structural mechanisms of gating and selectivity of human rod CNGA1 channel. Neuron 109(8), 1302-1313. http://doi.org/10.1016/j.neuron.2021.02.007.
Yau KW et al. (1977). Light-induced fluctuations in membrane current of single toad rod outer segments. Nature 269(5623), 78-80. http://doi.org/10.1038/269078a0.
Yau KW, Nakatani K (1984). Electrogenic NaCa exchange in retinal rod outer segment. Nature 311(5987), 661-663. http://doi.org/10.1038/311661a0.
Yau KW, Nakatani K (1985). Light-induced reduction of cytoplasmic free calcium in retinal rod outer segment. Nature 313(6003), 579-582. http://doi.org/10.1038/313579a0.
Yee R, Liebman PA (1978). Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. Journal of Biological Chemistry 253(24), 8902-8909. http://doi.org/10.1016/s0021-9258(17)34263-1.
Yoshikami S, Hagins WH (1971). Light, calcium and the photocurrent in vertebrate rods and cones. Biophysical Society Annual Meeting Abstracts 11, 47a.
