Peak isometric force (Po) normalised to cross-sectional area (CSA), termed specific force (SF), reflects muscle contractile quality. An age-related decrease in SF has been reported inconsistently at the myofilament level in human skeletal muscle. This has been attributed to both varying physical activity and health status between elderly cohorts and to methodological differences between research groups studying chemically skinned skeletal muscle fibres. Notably, the different activating solutions used (Kalakoutis et al 2021). To address these two issues the present study aimed to compare single fibre SF from both physically active master cyclists (MCs), elderly hip fracture patients (HFPs) and healthy young controls (YCs) using two different activating solutions to determine if this would reveal further insights into ageing and exercise effects on skeletal muscle.
Needle biopsy samples were obtained from the quadriceps of YCs (n = 6, age 26 ± 2) and MCs (n = 5, age 75 ± 1), and surgical biopsy samples from HFPs (n = 5, age 75 ± 4). Skinned fibre isometric force was measured (at 15oC, sarcomere length 2.75μm, pCa 4.5) in each fibre using activating solutions (A and B) which differed in their pH buffer. Solution A used Imidazole and Solution B used TES pH buffer. Fibres were then characterised based on myosin heavy chain (MHC) isoform composition. Po was normalised to CSA calculated assuming an elliptical shape and also to myosin protein content.
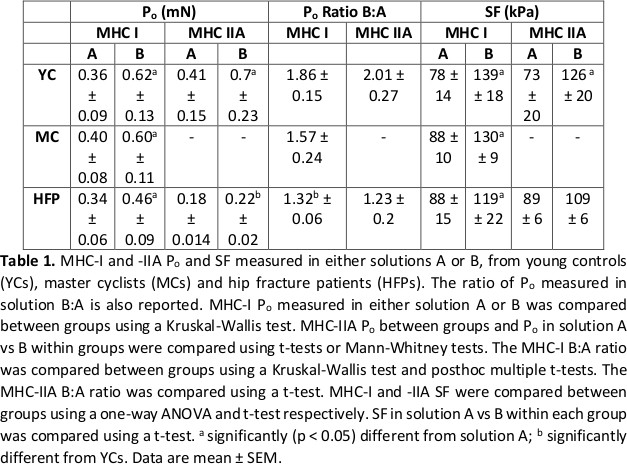
The data are summarised in Table 1. In MHC-I fibres Po and SF were significantly (p < 0.05) greater in solution B than A in YCs (n = 45 fibres), MCs (n = 72) and HFPs (n = 72), whilst in MHC-IIA fibres, Po and SF were only greater in solution B in YCs (n = 45). Between groups, SF was similar in both solutions, as was fibre myosin content. However, a difference was observed when the ratio between Po in solutions B:A was compared. The ratio was significantly (p < 0.05) greater in MHC I fibres from YCs (1.86) compared to HFPs (1.32), but not compared to MCs (1.57).
The present study has demonstrated that human skinned fibre force is highly sensitive to the chemical constituents of activating solutions used and did not identify any differences in SF between the three participant groups in either activating solution. However, the differing responses to the two solutions within the groups suggests that using two different activating solutions might be used to probe for functional differences occurring at the myofilament level between participants which may not be observed when only a single activation approach is used.
Biomedical Basis of Elite Performance 2022 (University of Nottingham, UK) (2022) Proc Physiol Soc 49, PC26
Poster Communications: Specific force of single, permeabilised, human skeletal muscle fibres studied using two activation strategies from healthy young, healthy older cyclists and elderly hip fracture patients.
Michaeljohn Kalakoutis1,2, Ross Pollock1, Norman Lazarus1, Marc George3, Onur Berber4, Roger Woledge1, Julien Ochala1,5, Stephen Harridge1
1 Centre for Human and Applied Physiological Sciences, School of Basic & Medical Biosciences, King's College London, London, England, United Kingdom 2 Randall Centre of Cell & Molecular Biophysics, School of Basic & Medical Biosciences, King's College London, London, England, United Kingdom 3 Guy's and St Thomas' NHS Foundation Trust 4 Royal Free NHS Foundation Trust 5 Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.