
Physiology News Magazine
Marvellous middle ears:
Structure and function in some unusual mammals
Features
Marvellous middle ears:
Structure and function in some unusual mammals
Features

https://doi.org/10.36866/pn.126.16
Professor Matthew J Mason
University of Cambridge, UK
Bridging the gap between the external ear canal and the cochlea, the middle ear apparatus has long attracted a level of interest from biological scientists disproportionate to its small size. Its development requires all three germ layers, and involves the neural crest. The possession of three middle ear ossicles – malleus, incus, and stapes – is a defining characteristic of mammals, and the discovery that the malleus and incus evolved from the jaw bones of ancestral vertebrates is regarded as a triumph of comparative anatomy. Exactly how the middle ear is constructed can also help us to determine what an animal can hear. This link between structure and function forms the basis of my research.
Beyond drawing pins and wheelbarrows: How the middle ear really works
Vertebrate inner ears are filled with fluid, which creates a problem for the detection of sound travelling in air. Impedance (strictly, specific acoustic impedance) is the ratio of pressure to velocity: a high impedance means that you need to apply a high pressure to a structure in order to move it. Because air has a lower impedance than the much denser cochlear fluids, most of the energy in sound would reflect back from the air–liquid interface if the sound were to impinge upon those fluids directly. A similar impedance mismatch means that it is difficult to hear someone talking if your head is underwater.
The middle ear is used as a way of reducing the impedance mismatch so as to improve sound energy transmission. The impedance of the thin, flexible tympanic membrane is close to that of air; thus, more of the sound energy is absorbed and less reflected than if the sound reached the cochlea directly. Vibrations of the eardrum in turn vibrate the ear ossicles, which are suspended within the air-filled middle ear cavity. The stapes footplate moves like a piston within the oval window, the entrance to the inner ear, conveying the sound vibrations through to the cochlea.

Textbooks highlight two features of the middle ear that help with impedance-matching (Fig.1). One is the area ratio: the tympanic membrane is larger in area than the stapes footplate. This increases pressure at the footplate, like a drawing pin increases pressure at the sharp end. The other is the lever ratio: the processes of the malleus and incus are unequal in length. This increases the force (but decreases the velocity) at the stapes, just as it is easier to lift a load in a wheelbarrow when the load is closer to the wheel than the handles are. For many years, zoologists have measured the area and lever ratios of mammals, including fossil species, and have used them to calculate the efficiency of impedance matching by the middle ear as an index of auditory acuity.
Unfortunately, there are problems with this concept of how the middle ear works. One is that the response of the tympanic membrane and ossicles is highly frequency-dependent, as we know from laser interferometric experiments. Moving a step beyond the textbooks, the simplest models which take frequency into account suggest that, as for other vibrating systems, compliance should be the dominant factor affecting the low frequency response of the middle ear, while mass should be dominant at high frequencies (Mason, 2016a).
Compliance is the reciprocal of stiffness. It can be increased by increasing the volume of the middle ear cavity or by loosening the tympanic membrane and ligaments of the ossicular chain. Increasing compliance would be expected to improve low-frequency sound transmission. The mass of the middle ear relates to the size of the middle ear ossicles: the smaller the ossicles, the better we might expect sound transmission to be at high frequencies. However, the pattern of vibrations gets much more complicated at high frequencies, and it is not clear from experimental studies that high-frequency sound transmission is actually affected by ossicular mass. The limits to high-frequency hearing in mammals remain unclear, and very likely involve parts of the auditory system other than the middle ear.
Air heads
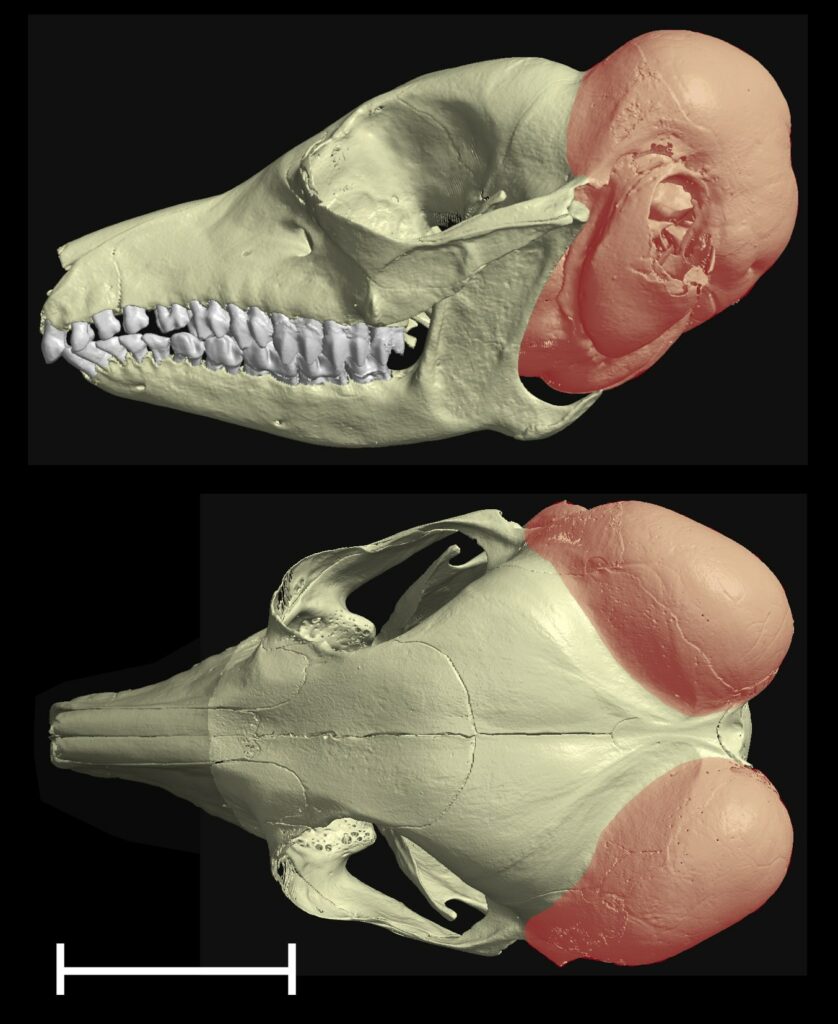
As a comparative physiologist, I look for similarities in how the middle ear has evolved in different groups of animals, and how they might suggest common evolutionary selective pressures. One striking pattern, first noted in the 19th century, is that small desert mammals such as gerbils and kangaroo rats tend to have very large middle ear cavities relative to the sizes of their skulls. This is taken to an extreme in the elephant shrew (Macroscelides flavicaudatus), in which the volume of the two air-filled cavities added together exceeds the volume of the brain (Mason, 2016a). The middle ear cavity in most mammals (humans are an exception) is contained within a bony swelling on the basicranium known as the auditory bulla. In Macroscelides, each bulla wraps all around the posterior skull to reach even the dorsal aspect (Fig.2).
Michael Ravicz and John Rosowski (1997) have shown that having a large middle ear cavity volume increases acoustic compliance and improves low-frequency sound transmission (<3 kHz) in gerbils, which are known from behavioural studies to have excellent low-frequency hearing for their size. Elephant shrews have not yet been tested, but we would certainly expect that they would also be able to hear well at low frequencies. This ability may be especially important in desert environments, because lower-frequency sound travels further in dry air (Huang et al., 2002). Having good low-frequency hearing could help animals in dispersed populations communicate with each other, or detect predators from further away. Large animals have no trouble hearing low frequencies because of their large middle ears, but because acoustics depends on absolute dimensions, small mammals need to fit relatively enormous middle ears into their small skulls in order to achieve the same feat.

Going underground
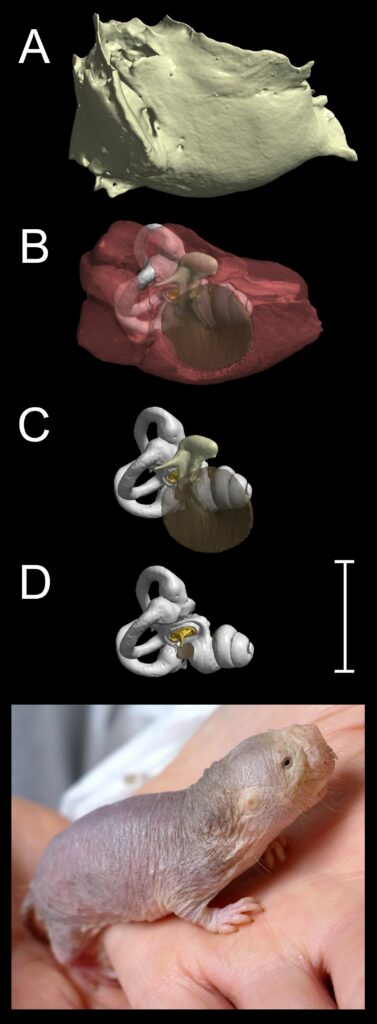
Caliban’s plea to his companions, “Pray you, tread softly, that the blind mole may not hear a foot fall”, drew on a longstanding belief that moles must hear very well to make up for their poor vision. In fact, European moles have eyes hidden in their fur and are not blind (Fig.3). As for their hearing, studies of subterranean mammals (mostly mole-rats, to be fair to Shakespeare) have shown them to have unusually poor auditory acuity when tested under experimental conditions, even at the low frequencies of a few hundred Hertz, which propagate best in underground tunnels. Unlike gerbils and elephant shrews, subterranean mammals such as the naked mole-rat (Heterocephalus glaber, Fig.4) do not have grossly enlarged middle ear volumes. Heterocephalus has no pinna, a very narrow ear canal, flimsily constructed ossicles and no stapedius muscle. Turning to the inner ear, it has recently been shown that this species has abnormal outer hair cells and no cochlear amplifier (Pyott et al., 2020).
Nearly half a century after The Tempest was written, Edward Topsell commented that moles “dig about their lodging long passages, which bringeth noises and voices to them, being spoken never so low and softly, like as the voice of a man carryed in a trunk, reed or hollow thing”. This has become known in the much more recent literature as the “stethoscope effect”. It has been suggested that the stethoscope effect might compensate for the poor hearing of subterranean mammals; i.e. these animals would not need acute audition if sound is amplified within the burrows that they normally inhabit (Lange et al., 2007). I remain to be convinced by this hypothesis, but it is an intriguing idea.
Good vibrations
So, if moles are indeed very sensitive to foot-falls, must that be because of a stethoscope effect of their burrow systems? Not necessarily. Foot-falls result in vibrations propagating in air, which is what we would normally call “sound”, but they also cause seismic vibrations to propagate in the soil. Ground vibrations might in principle be picked up by the somatosensory system. Talpid moles have specialised somatosensory structures called Eimer’s organs on their noses, and disproportionately large representations of both noses and forefeet in the somatosensory cortex (Catania, 2000).
The somatosensory system is not the only means of detecting seismic vibrations. Vibrations applied directly to the skull can be conveyed to the cochlea through a number of possible routes collectively known as “bone conduction”. In human medicine, bone conduction is used as a diagnostic test for conductive hearing loss. A vibrating tuning fork is applied to the skull of a person who appears to be deaf: if the person can hear it, the cochlea and neural pathways must be intact, suggesting that it may be the middle ear that is damaged.
Although it remains unclear whether talpid moles and mole-rats detect ground vibrations primarily through their somatosensory or auditory systems, one group of subterranean mammals has very striking adaptations of the middle ear that strongly suggest that they use a form of bone-conducted hearing. These are the golden moles of southern Africa (Chrysochloridae), some of which have relatively enormous mallei. The malleus of the mouse-sized desert golden mole Eremitalpa granti, for example, is twice the mass of its human equivalent (Mason et al., 2006) (Figure. 5) In normal hearing, airborne sound sets the ossicles vibrating, leading to relative movement between stapes and inner ear. This vibrates the cochlear fluids, which activates the hair cells. The mallei of golden moles are so large and dense, and their eardrums so small, that airborne sound would not be expected to result in much ossicular movement. However, if the head is in firm contact with the ground, the skull will be set into motion by low-frequency seismic vibrations. The inertia of the huge ear ossicles will tend to result in them staying in one place, with the head vibrating around them, leading to the relative movement between stapes and inner ear that is required for hearing (Mason, 2003). Field studies in Namibia have shown that desert golden moles – which really are blind – are able to navigate from one grassy clump to the next in their search for food. It has been proposed that ground vibrations produced as wind passes through the grass, measured at around 30 dB above background noise at 300 Hz, allow the clumps to act as “seismic beacons”, which the moles can detect and orient towards, using their massive mallei (Narins et al., 1997).

Middle ear frontiers
Although we are learning more all the time about hearing, studies tend to focus on certain model species. These include aridregion rodents such as gerbils and chinchillas, since their capacious middle ears offer easy surgical access. How the middle ear works in other species often remains to be tested experimentally, especially when it comes to subterranean mammals. Surprisingly, big questions remain about human middle ears too. For example, what is the function of the tensor tympani muscle? It is not required to tense the tympanic membrane, as its name suggests, since several mammals lack this muscle and still have perfectly tense eardrums. By taking a comparative perspective, we can see what is necessary and sufficient for hearing, better appreciate the similarities and differences between human ears and those of model species, and develop a deeper understanding of how structure links to function.
Acknowledgements
The Macroscelides reconstructions were made from specimen CAS MAM 30152, California Academy of Sciences, San Francisco, collected by Galen Rathbun. The Heterocephalus reconstructions were made from a specimen kindly provided by Ewan Smith, University of Cambridge, UK.
References
Catania KC (2000). Cortical organisation in Insectivora: The parallel evolution of the sensory periphery and the brain. Brain, Behavior and Evolution 55(6), 311-321. https://doi.org/10.1159/000006666.
Huang GT et al. (2002). Mammalian ear specializations in arid habitats: Structural and functional evidence from sand cat (Felis margarita). Journal of Comparative Physiology A 188(9), 663-681. https://doi.org/10.1007/s00359-002-0332-8.
Lange S et al. (2007). Living in a “stethoscope”: burrow acoustics promote auditory specializations in subterranean rodents. Naturwissenschaften 94(2),134-138. https://doi.org/10.1007/s00114-006-0168-0.
Mason MJ (1999). The Functional Anatomy of the Middle Ear of Mammals, with an Emphasis on Fossorial Forms. PhD Thesis, University of Cambridge, UK.
Mason MJ (2003). Bone conduction and seismic sensitivity in golden moles (Chrysochloridae). Journal of Zoology 260(4), 405-413. https://doi.org/10.1017/S0952836903003868.
Mason MJ (2016a) Structure and function of the mammalian middle ear. I: Large middle ears in small desert mammals. Journal of Anatomy 228(2), 284-299. https://doi.org/10.1111/joa.12313.
Mason MJ (2016b) Structure and function of the mammalian middle ear. II: Inferring function from structure. Journal of Anatomy 228(2), 300-312. https://doi.org/10.1111/joa.12316.
Mason MJ et al. (2006). Ossicular density in golden moles (Chrysochloridae). Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology 192(12), 1349-1357. https://doi.org/10.1007/s00359-006-0163-0.
Narins PM et al. (1997). The use of seismic signals by fossorial southern African mammals: A neuroethological gold mine. Brain Research Bulletin 44(5), 641-646. https://doi.org/10.1016/s0361-9230(97)00286-4.
Pyott SJ et al. (2020). Functional, morphological, and evolutionary characterization of hearing in subterranean, eusocial African mole-rats. Current Biology 30(22), 4329-4341. https://doi.org/10.1016/j.cub.2020.08.035.
Ravicz ME, Rosowski JJ (1997). Sound-power collection by the auditory periphery of the Mongolian gerbil Meriones unguiculatus: III. Effect of variations in middle-ear volume. Journal of the Acoustical Society of America 101(4), 2135-2147. https://doi.org/10.1121/1.418275.
