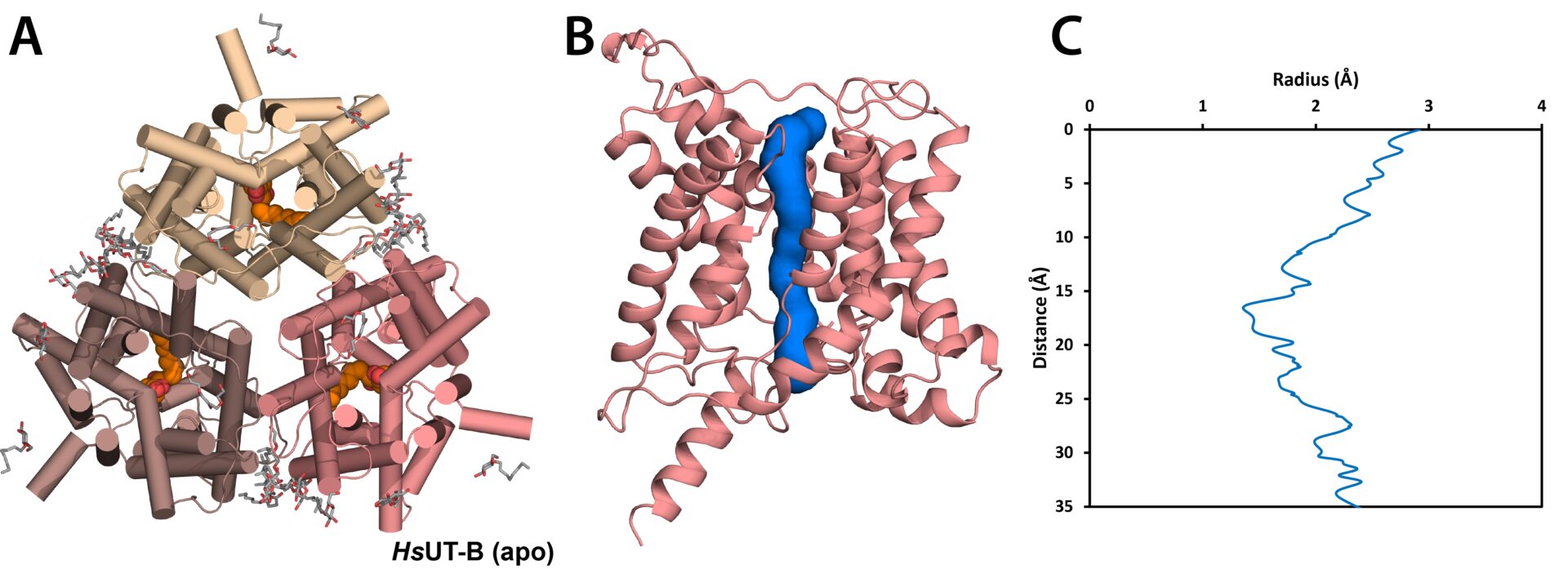
Introduction: Urea is a key molecule in several physiological processes including urea cycle and urine volume regulation (1). These functions require urea transport across the plasma membrane, which in humans is facilitated by two urea transporters, UT-A (also called UT-2 or SLC14A2) and UT-B (UT-1, SLC14A1) (1). UT-A isoforms are expressed in various compartments of the loops of Henle to reabsorb urea from glomerular filtrate to tubular cells and eventually to blood (1), thereby regulating urine volume via the osmotic gradient. In contrast, UT-B is widely expressed in human tissues and so is involved in a wider range of physiological processes (2). Additionally, UT-B has a unique clinical implication as the primary antigen for the Kidd blood group system (3), where its Asp/Asn amino acid variation at residue 280 determines its Jk blood group phenotype. These make UT-A and UT-B important targets not only from drug discovery perspective but also for clinical applications.
Aims: This study aimed to determine high-resolution structures of human UT-A and UT-B to understand structural aspects of their function, and to enable the development of therapeutics targeting these urea transporters.
Methods: Human urea transporters were expressed in HEK293 cells and purified in detergents for structural studies. Atomic-resolution (2.3 – 2.9 Å) structures of apo UT-A and UT-B, and inhibitor-bound UT-B, were determined by X-ray crystallography and cryo-electron microscopy. Computational tools such as APBS electrostatics, ICM-Pro and MOLE were used to analyse the proteins’ surface charges, inhibitor binding, and channel pore radius, respectively.
Results: In this talk, I present insights into the molecular mechanisms of human UT-A and UT-B function from our high-resolution structures of the proteins in apo and inhibitor-bound states. These structures reveal UT-A’s extracellular surface to be more negatively charged than UT-B, and that its surface charge can significantly vary within the pH ranges of 5.5 and 7.5 observed in the tubular lumen. This suggests that the function of UT-A may be regulated by the pH of the convoluted tubules, in contrast to UT-B. The divergent surface properties between the two transporters also establish the basis for structure-guided drug discovery programs which target each protein individually. Lastly, the position of UT-B’s antigenic surface raises interesting questions about the epitope targeted by anti-Jk antibodies.
Conclusion: The urea transporter structures further our physiological understanding of urea transport and advance efforts for therapeutic development programs targeting them.