Introduction: since the work of Hodgkin & Huxley, mathematical models of ion channel gating have been used to understand and predict the effects of ion currents in action potential formation. Today we still tend to use the same approach to voltage-clamp protocol design that Hodgkin & Huxley used: designs based on simple square waves, with large gaps to return to steady states, that enable model parameter values to be estimated manually from graph paper.
Aims/Objectives: to use short high-information voltage clamp protocols in partnership with computational modelling to characterise ion currents. To allow a model (Figure 1A) to be fitted, and tested as well as multiple experimental interventions in the same cell. Here we show results of using short protocols to capture the kinetics of IKr / KV11.1 / hERG currents in a range of settings.
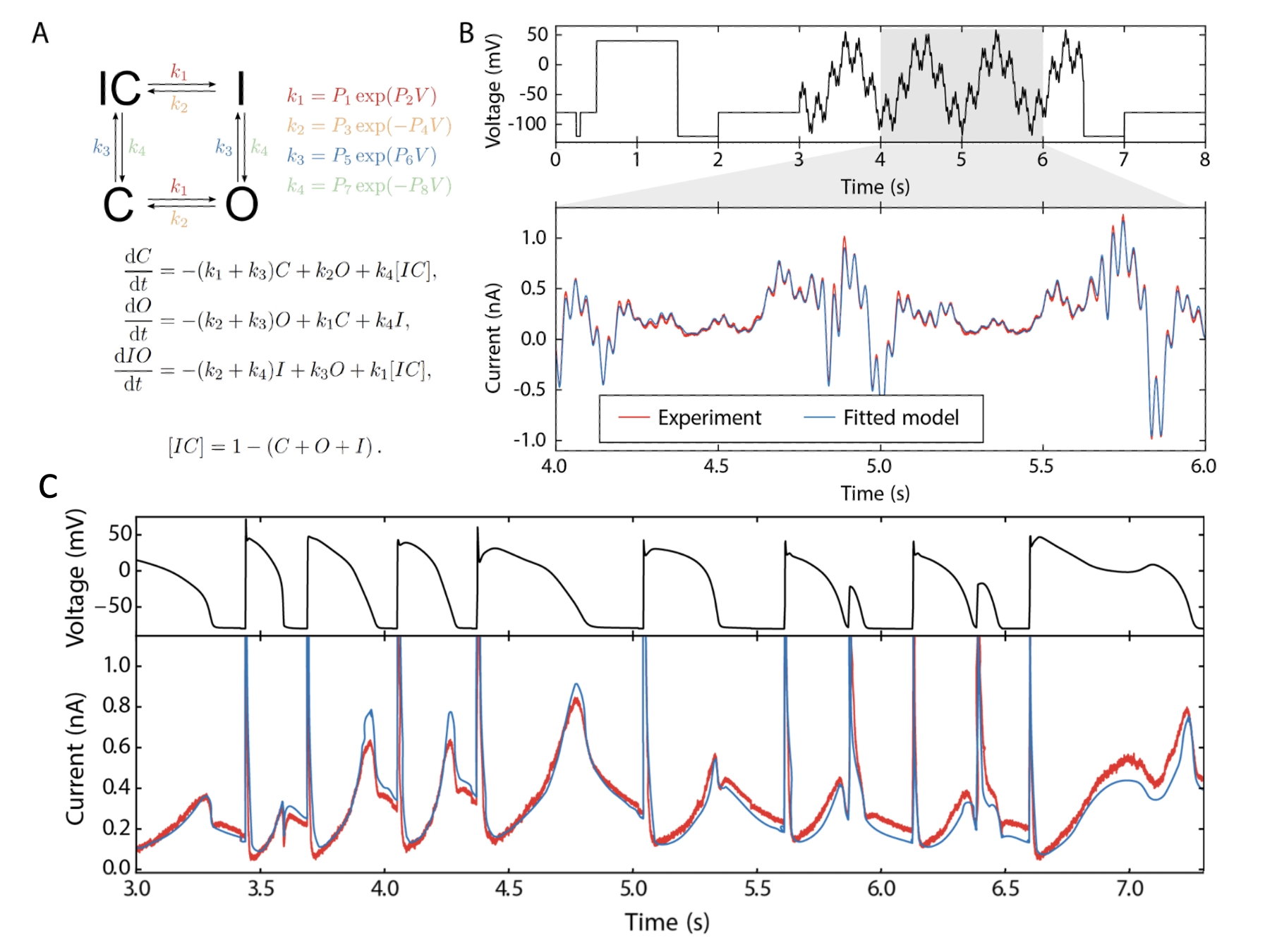
Method: we apply either a short sinusoidal voltage clamp protocol (Figure 1B, [1]) or a square wave ‘staircase’ version [2] to CHO cells stably expressing hERG1a at room temperature or physiological temperature in manual and automated patch settings. We then use computational optimisation to fit a simple mathematical model for hERG to the resulting currents, and use it to predict the results of conventional voltage clamp protocols and physiological action potentials.
Results: the short protocols result in highly predictive mathematical models: Figure 1C shows a prediction from a model fitted to the sinusoidal protocol shown in Figure 1B against experimental data. The parameter values within these models then capture our knowledge of channel gating more accurately than a series of Current-Voltage or Time Constant-voltage curves, with the benefit they can be re-used to predict currents in new situations/voltage-clamp protocols that were not examined in the original experiment. There are also opportunities to use mathematical models to account for patch clamp artefacts [3] to consolidate information from different patch clamp recordings more reliably.
Conclusions: this approach offers the opportunity to intervene and reassess currents multiple times in one experiment, and to generate a mathematical model that quantitatively captures our understanding about channel gating. For instance, we can alter temperature to examine the temperature dependence at the level of individual rate and voltage-dependence parameters within a model, rather than at the level of processes such as ‘activation’ or ‘recovery from inactivation’ [4]. We can also build models of mutant channels, and/or characterise changes in currents in the presence of drug compounds that alter channel gating [5].