Introduction
The kidney maintains blood pressure and electrolyte balance through the entwined actions of the tubular nephron segments and ion transporters. Recent investigations into rare monogenic diseases, specifically Gordon and Gitelman syndromes, occurring in the distal convoluted tubule (DCT) segment of the kidney, underline the critical physiological role of this segment as well as a DCT-specific membrane protein, sodium chloride cotransporter (NCC, SLC12A3), in regulating blood pressure. Despite its crucial significance, no well-characterized and independently validated human DCT cell lines have been identified, with only a limited number of murine DCT cell lines available for in vitro studies. To date, none have been incorporated into the Organ-on-a-Chip (OOaC) system. This study aims to employ a novel, immortalized human DCT cell line into a multi-channel OOaC culture system, with the ultimate goal of creating the first human DCT Tubule-on-a-chip (TOaC).
Method
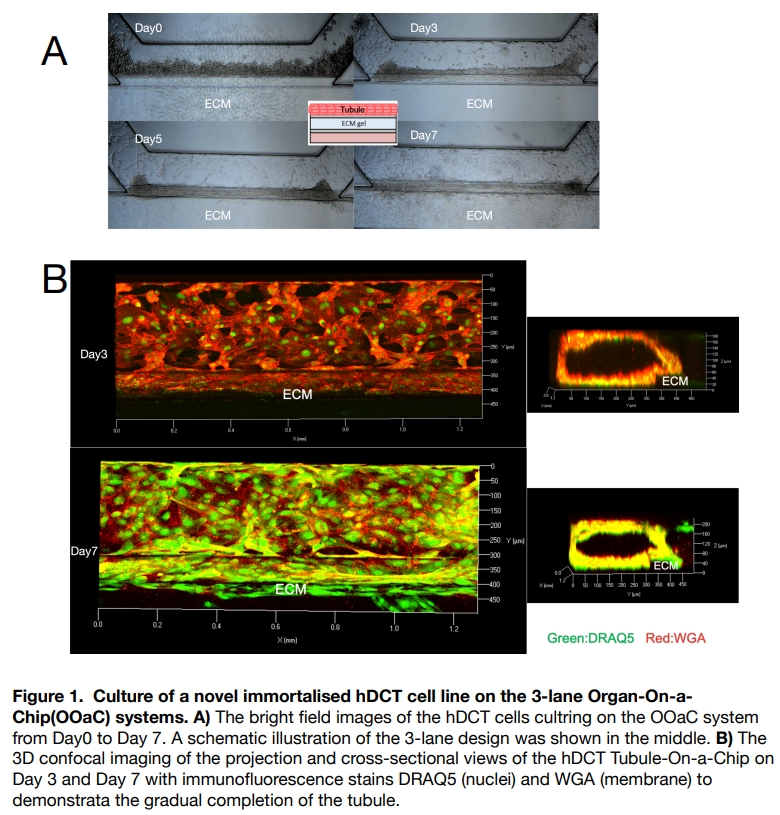
Immortalised hDCT cells characterised in our lab (gifted from Dr Kusaba, Kyoto Prefectural University of Medicine, Japan) were cultured as previously described (Ikeda et al., 2020). Cells were applied in a three-lane, micro-plate-based microfluidic chip platform OrganoPlate (Mimetas, Leiden, Netherland) following manufacture’s protocol with modifications on the constitution of the extracellular matrix gel. Tubules formed in the OrganoPlate channel on an average of 5-7 days of culture. TOaCs were ï¬xed using 4% w/v formaldehyde-PBS for 15min at room temperature or lysed with TRI reagent for RNA isolation. Segment specific marker expression was confirmed by staining with fluorescently tagged antibodies/lectins and qPCR.
Result
Barrier integrity assay in the OrganoPlate using fluorescent probes confirmed tight junctions between cells. qPCR of the cell lysate showed these TOaCs expressed the DCT-specific marker NCC (SLC12A3). NCC antibody showed positive staining localised to the apical membrane.
Discussion
These preliminary data demonstrate that this novel hDCT cell line is able to form tubule-like structures in the OrganoPlate. Given this cell line were derived from primary human DCT cells, we propose this TOaC model will better reflect in vivo human DCT physiology, especially useful in exploring the functionality of membrane transporters and replicating the fluidic movement in the kidney, comparing to conventional iPSC-based systems. Tubular function will be validated by ion transport assays and pharmacological responses. In the future, we aim to create patient-specific TOaC from urine-derived cells and conduct therapeutic optimisation, thereby bringing true personalised medicine to nephrology.