
Dr Svetlana Mastitskaya, BHF Intermediate Basic Science Research Fellow at University College London
With a PhD in cardiac regeneration and several years of research in the field of Cardiovascular Neuroscience, I’ve always been fascinated by bidirectional interactions between the brain and the heart, and how these interactions are coordinated by the vagus nerve. My research is focused on neural and humoral (bloodborne) mechanisms of coronary blood flow regulation in health and disease. I’m curious as to how these mechanisms can be manipulated to improve cardiovascular health.
The wonders of the wanderer
The vagus nerve, also known as the wanderer due to its extensive distribution and complex path, plays a vital role in regulating various bodily functions. As the main peripheral nerve of the parasympathetic branch of the autonomic nervous system, it controls heart rate, blood pressure, digestion, respiration, and immunity. Vagus nerve stimulation is used for drug-resistant depression and epilepsy, and has potential for treatment of hypertension, heart failure, and cardiac arrhythmias. Through my research, I have demonstrated the crucial importance of the vagus nerve in protecting the heart during a heart attack, preserving cardiac function in heart failure and improving exercise capacity (Mastitskaya et al., 2012; Machhada et al., 2020).
In heart failure, the autonomic balance is shifted towards upregulation of the sympathetic nervous system to compensate for the reduced functional capacity of the damaged heart and maintain an adequate blood supply to the body. However, these alterations become detrimental in the long term, overriding vagal activity and promoting maladaptive cardiac remodeling.
Vagus nerve stimulation is known to reduce the heart rate, reduce susceptibility of the myocardium to malignant arrhythmias and oppose the effects of sympathetic system overactivation in general, which improves survival. If the vagus nerve is stimulated acutely during a heart attack, it dramatically reduces the infarct size (death of the heart tissue due to inadequate blood supply). The question is, how does it work?
Time is muscle; muscle is life
Time is critical in a heart attack. Within 80-90 minutes of halted blood supply, the heart muscle starts dying, and after six hours most of the affected heart tissue is irreparably harmed. Promptly removing the blockage in the culprit artery is vital to prevent cardiac tissue death. However, in as many as 40% of cases, reopening the artery does not fully restore blood flow on the microvascular level, leading to the detrimental “no-reflow” phenomenon. No-reflow heavily contributes to the poor healing of the infarct, which can lead to heart failure, malignant arrhythmias and even cardiac rupture. Fortunately, vagus nerve stimulation limits infarct size and prevents no-reflow, offering potential solutions (Mastitskaya et al., 2012; Uitterdijk et al., 2015).
Brain-gut-heart axis
The vagus nerve’s influence extends to the abdomen, where approximately 90% of its efferent fibers (carrying impulses away from the central nervous system) are found, regulating digestive tract motility and the production of gut hormones that are essential for energy homeostasis. Among these hormones, glucagon-like peptide-1 (GLP-1) exhibits substantial positive effects on the cardiovascular system, including vasodilation, reduced blood pressure, improved myocardial blood flow, and cardiomyocyte survival.
Stimulation of the vagus nerve can thus indirectly protect the heart during an acute heart attack due to an increased release of GLP-1 from the gut (Basalay et al., 2016; Mastitskaya et al., 2016). In emergency scenarios where direct vagus nerve stimulation is impractical, downstream activation of GLP-1 receptors can trigger a protective pathway for the heart.
A tight squeeze: pericytes and cardiac health
The intricate capillary network within the myocardium plays a pivotal role in meeting the heart’s ever-changing metabolic demands. Capillaries consist of endothelial cells forming the capillary tube, surrounded by pericytes. Pericytes are contractile cells extending their processes around and along the capillaries. They play key roles in maintaining vascular integrity and mediating regeneration of both the vasculature and cardiac tissue.
Pericytes express receptors to various vasoactive substances, hormones and neurotransmitters and thus can regulate the capillary blood flow in response to metabolic and neural stimuli (O’Farrell & Attwell, 2014). In ischaemia (decreased blood flow and oxygen to the heart muscle), pericytes contract and constrict the underlying capillaries. If the ischaemia is prolonged (as in a heart attack) pericytes may die in rigor, and the capillaries remain permanently constricted (O’Farrell et al., 2017). Notably, pericyte contraction during ischemia can be reversed using pericyte-relaxing agents such as adenosine and GLP-1, offering a potential avenue for preventing no-reflow phenomenon and improving the outcomes of heart attack by targeting the microvasculature.
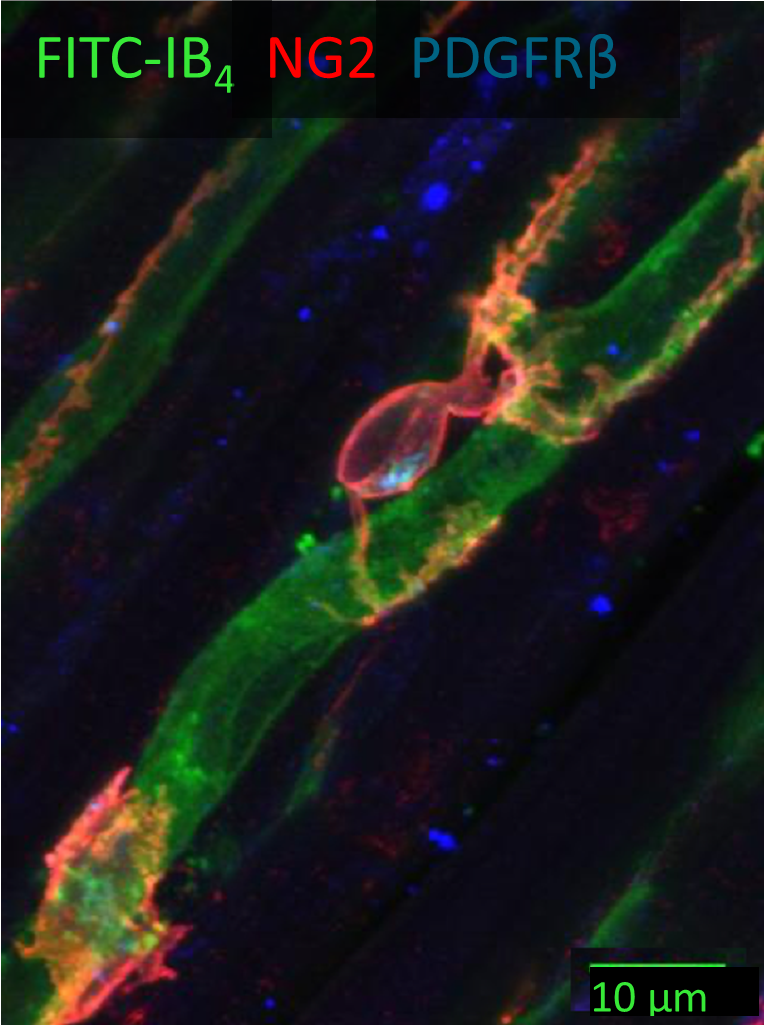
Fine-tuning cardiac health through modulation of cardiac pericyte function and selective stimulation of the vagus nerve
Investigating the regulation of coronary blood flow through autonomic control of cardiac pericyte function is the main focus of my research. However, I have several ongoing projects where I’m delving into preventing pericyte constriction during ischemia, elucidating the role of cardiac pericytes in diabetic cardiomyopathy and Alzheimer’s disease-related cardiac complications, and modulating cardiac pericyte function to enhance exercise capacity.
I use pharmacological and optogenetic (a technique to control the activity of neurons using light and genetic engineering) approaches to modulate the activity of the autonomic nervous system and understand how the vagus nerve affects the function of cardiac pericytes. I am also developing technologies that specifically target the fibers within the vagus nerve to improve heart health. Continuing to explore the connection between the brain and the heart can help us understand what goes wrong to better protect hearts from breaking.

