Introduction: Spatial heterogeneity of action potential duration (APD) is key to arrhythmia generation in the ventricle1,2. Our previous intact heart studies have suggested that earlier-activating regions of the ventricle have longer APD at baseline and show exaggerated response to IKr blockade, thereby contributing to increased spatial APD heterogeneity. However, it is unclear whether these effects result from differences in intrinsic cardiomyocyte properties between earlier and later activating ventricular regions, or from electrotonic coupling between neighbouring cardiomyocytes and Purkinje cells3. A series of combined optical mapping and cardiomyocyte isolation experiments were, therefore, performed to compare differences in intrinsic APD between cardiomyocytes isolated from earlier and later activating ventricular regions.
Objective: To determine whether the intact heart activation sequence influences the APD of ventricular cardiomyocytes.
Methods: All procedures involving animals were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 under project licence PP5254544. Male New Zealand White rabbit hearts (N=6) were Langendorff-perfused with oxygenated Tyrode’s solution (37±0.5°C) containing FluoVolt and blebbistatin. Optical signals reflecting transmembrane voltage were recorded from the anterior epicardial surface of the hearts, then activation time maps constructed. Cardiomyocytes were then enzymatically isolated from five regions of the left and right ventricles with distinct activation times. Isolated cardiomyocytes were loaded with FluoVolt, then fluorescence traces reflecting transmembrane voltage were recorded from single cardiomyocytes by an investigator blinded to the origin of each cardiomyocyte group. The effect of activation time on APD90 in the intact heart was quantified for each heart using simple linear regression analysis. The effect of intact heart activation time on isolated cardiomyocyte APD90 was assessed using a linear mixed-effects model with unstructured covariance matrix. ‘Animal’ was included as a grouping variable with random intercept, and ’activation time’ and ‘ventricle’ were included as fixed effects.
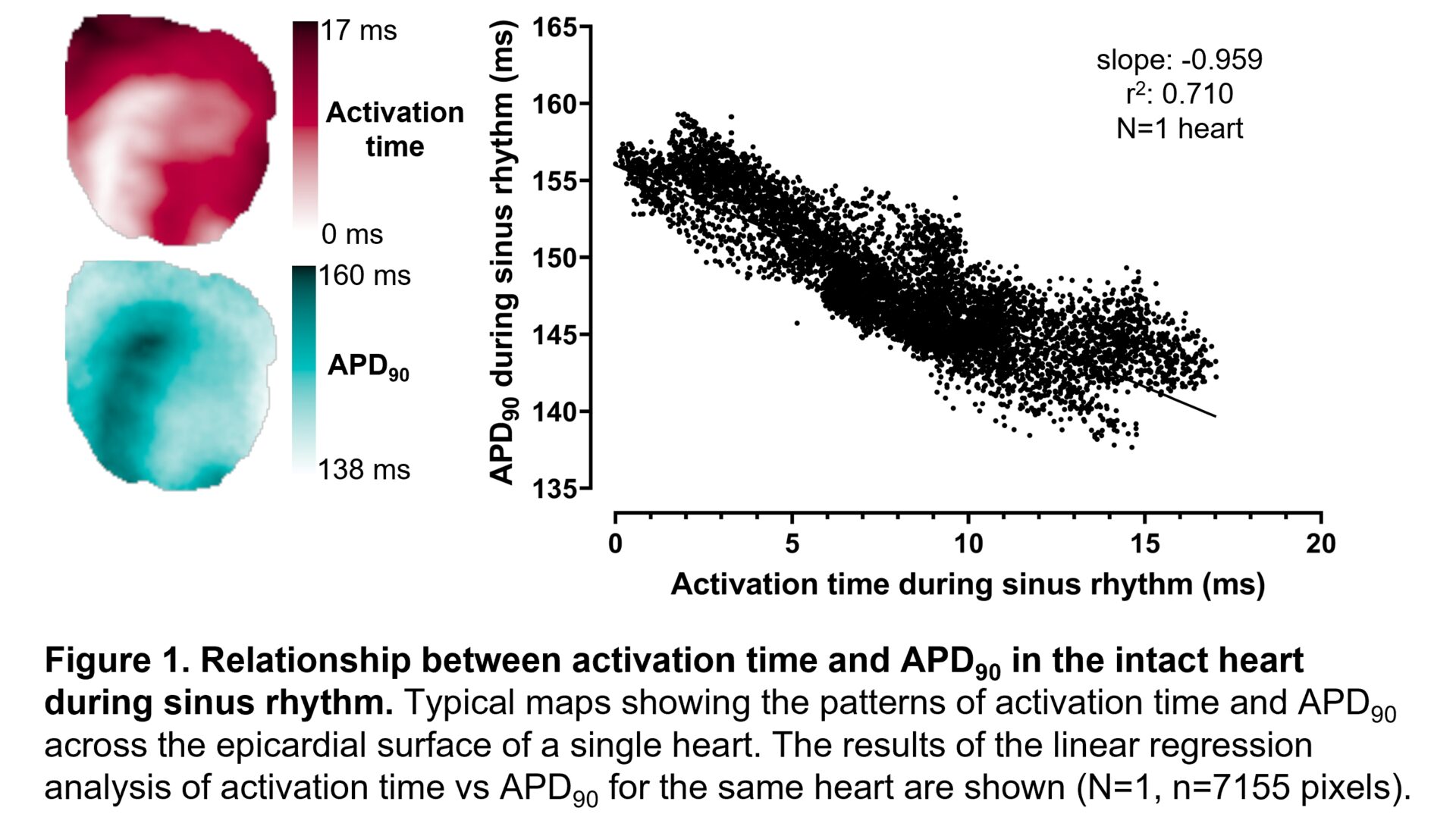
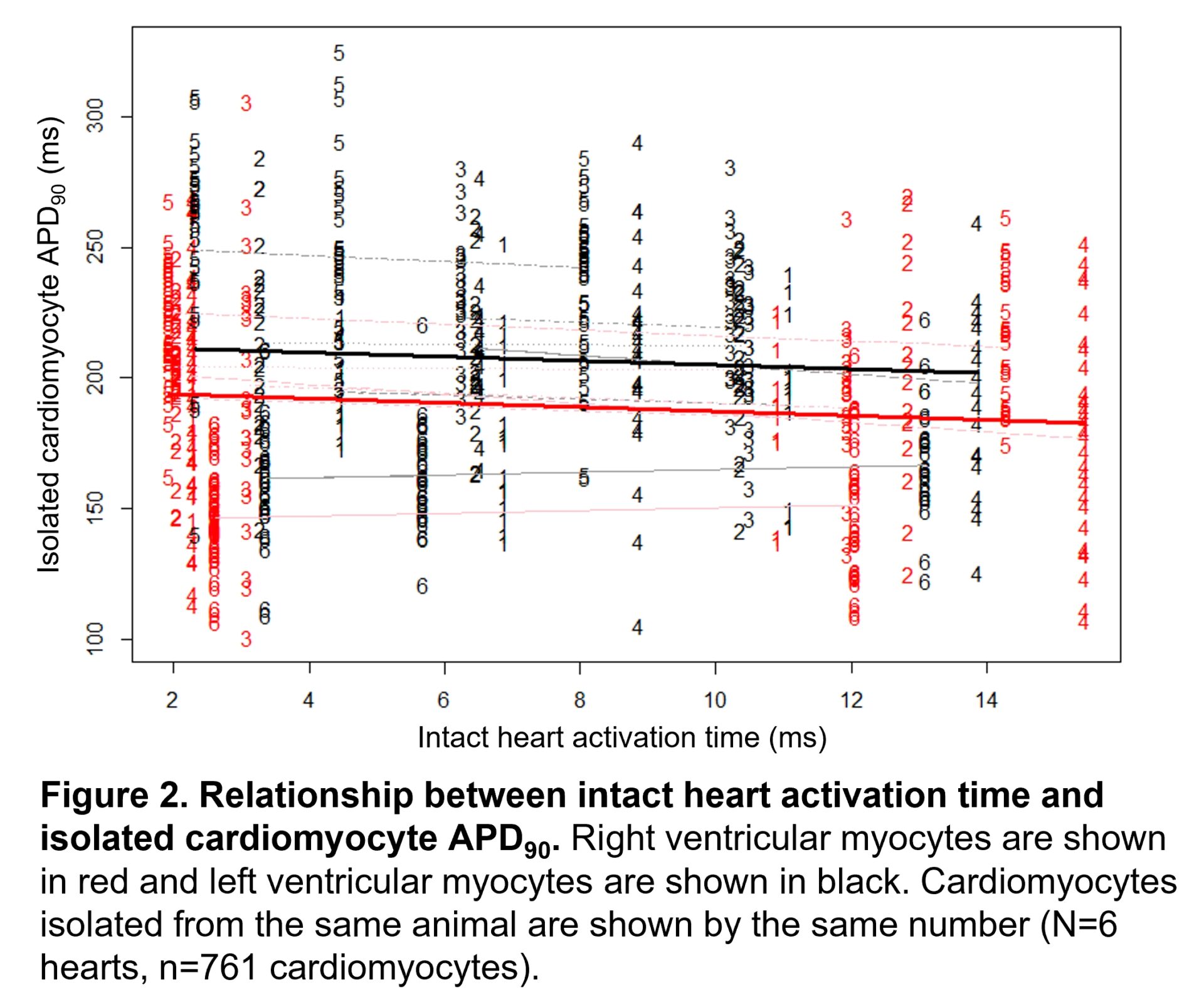
Results: A consistent inverse relationship between activation time and APD90 was observed in the intact heart during sinus rhythm (mean slope: -0.865±0.132, mean r2:0.602±0.084; N=6 hearts). Linear mixed-effects analysis revealed significant evidence for a main effect of intact heart activation time on APD90 of isolated cardiomyocytes (slope estimate: -0.814, 95% CI: -1.327 to -0.300, P=0.0020). There was also significant evidence for a main effect of ventricle on APD90, with shorter APD90 detected among RV myocytes than LV myocytes (slope estimate: -17.611, 95% CI: -22.011 to -13.211, P<0.0001).
Conclusion: These findings suggest that the intact heart activation sequence may influence APD at both the intact heart and cardiomyocyte level, such that cardiomyocytes located within earlier activating regions of the ventricle have longer APD.