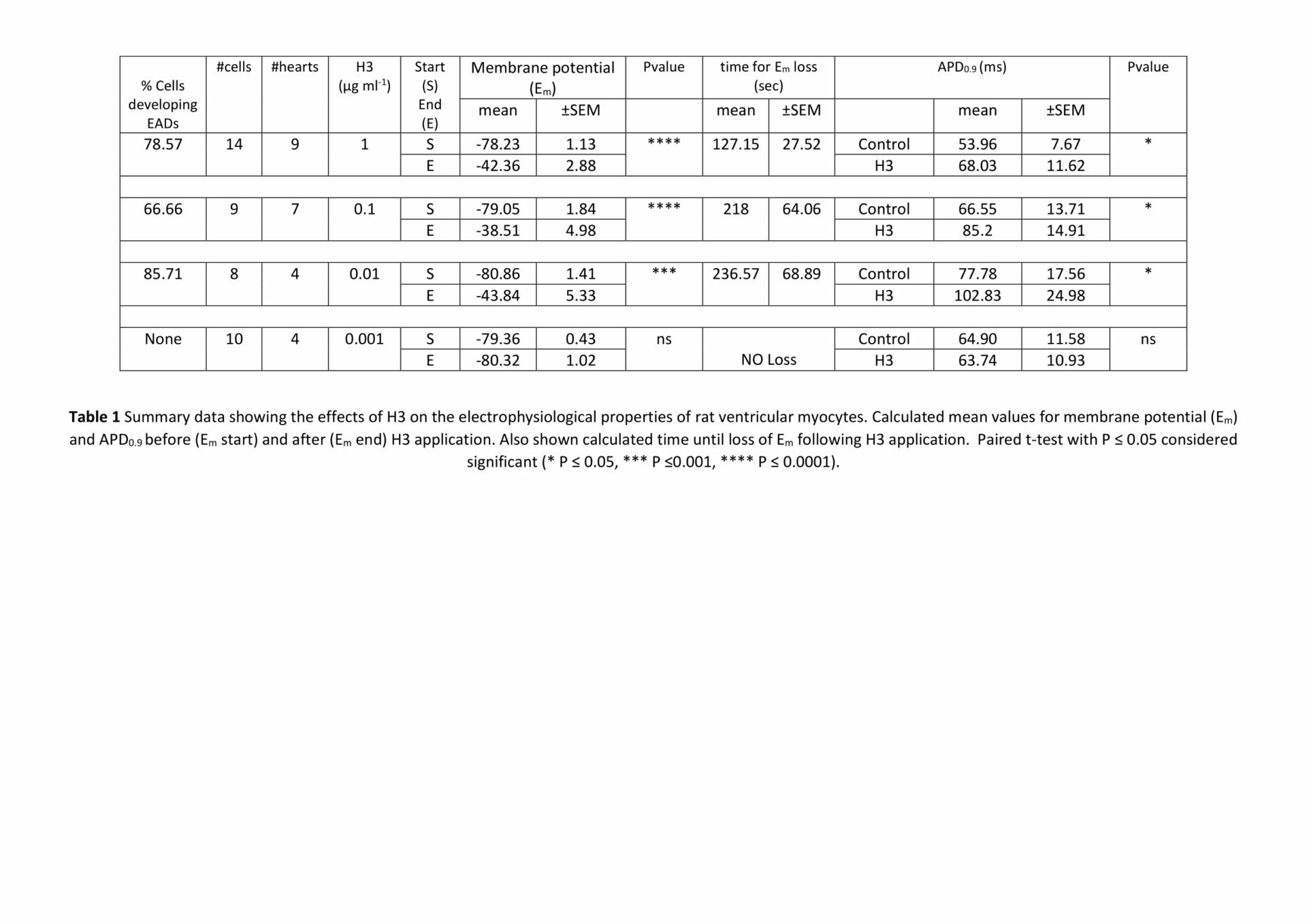
Histones are highly basic proteins present in eukaryotic cell nuclei. DNA wraps around histones creating structural units called nucleosomes, the building blocks of chromatin1. Previous work2 has shown that following sepsis or blunt trauma, circulating histones released from damaged cells cause secondary damage to organs, including the heart. Here we investigated the mechanism of H3 histone toxicity in isolated ventricular myocytes. Wistar rats (150–200 g) were sacrificed in accordance with the UK Home Office Guidance on the Operation of Animals (Scientific Procedures) Act of 1986. H3 histone (5 µg/ml) induced membrane damage and cell necrosis. Following 10 min exposure, topographical images obtained using scanning ion conductance microscopy’ (SICM) showed fine dark lines consistent with ‘micro-tears’. The effects of H3 on membrane potential were assessed using whole cell current clamp recording. Under control conditions, the mean (± SEM) resting membrane potential (Em) was -79.1 ±0.65 (n=87). H3 (µg/ml) application caused a dose and time dependent depolarisation of Em, spontaneous action potentials and the development of early afterdepolarisations (EADs, Table 1). Low levels of H3 (0.1-0.01 μg/ml) increased the action potential duration measured 90% of maximal amplitude (APD0.9; Table 1). These data show that H3 induces membrane damage, leading to myocyte depolarisation and pro-arrhythmic changes in the action potential. Such effects may contribute to cardiac dysfunction in pathological conditions that result in a rise in circulating histones.
Physiology 2023 (Harrogate, UK) (2023) Proc Physiol Soc 54, PCB002
Poster Communications: Electrophysiological effects of extracellular histones in isolated cardiomyocytes
Moza Al-Owais1, Z Yang1, H Kirton1, E White1, D Steele1,
1School of Biomedical Sciences, University of Leeds, Leeds LS2 9JT, UK Leeds United Kingdom,
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.