Bone development and remodeling are controlled by the phosphoinositide-3-kinase (PI3K) signaling pathway. We investigated the effects of downregulation of phosphatase and tensin homolog (Pten), a negative regulator of PI3K signaling, in osteoprogenitor cells. Using a mouse model with Pten deficiency in preosteoblasts, we aimed to identify mechanisms that are involved in the regulation of bone turnover and are linked to bone disorders.
Animal experiments were approved by local authorities (Regierungspräsidium Leipzig, Germany (TVV30/19 and TVV32/17). Bone marrow stem cells (BMSCs) were isolated from inducible conditional Pten knockout (Pten cKO) mice with a deletion of Pten exon 5 in Osterix/Sp7 expressing osteoprogenitor cells (1). Femora, tibiae and BMSCs from Pten cKO and Cre negative control mice were compared. Expression of osteogenic markers, Pten protein and AKT phosphorylation was determined. Bone phenotyping was performed by µCT and 3-point bending test. Number of osteoclasts and osteoblasts was determined by tartrate resistant acid phosphatase immunohistochemistry. Proliferation of BMSCs was measured by counting nuclei and Ki-67-stained cells. In vitro adipogenic and osteogenic differentiation was determined by Nile Red and alkaline phosphatase staining, respectively, as well as detecting gene expression changes in adipogenic and osteogenic markers. Bone turnover was assessed by ELISA detecting procollagen type 1 amino-terminal propeptide (P1NP) and C-terminal telopeptide (CTX). Measurements were converted to log fold changes±SD and normalized to the mean of control animal values. Significant differences were determined by one-sample t-test (p<0.05).
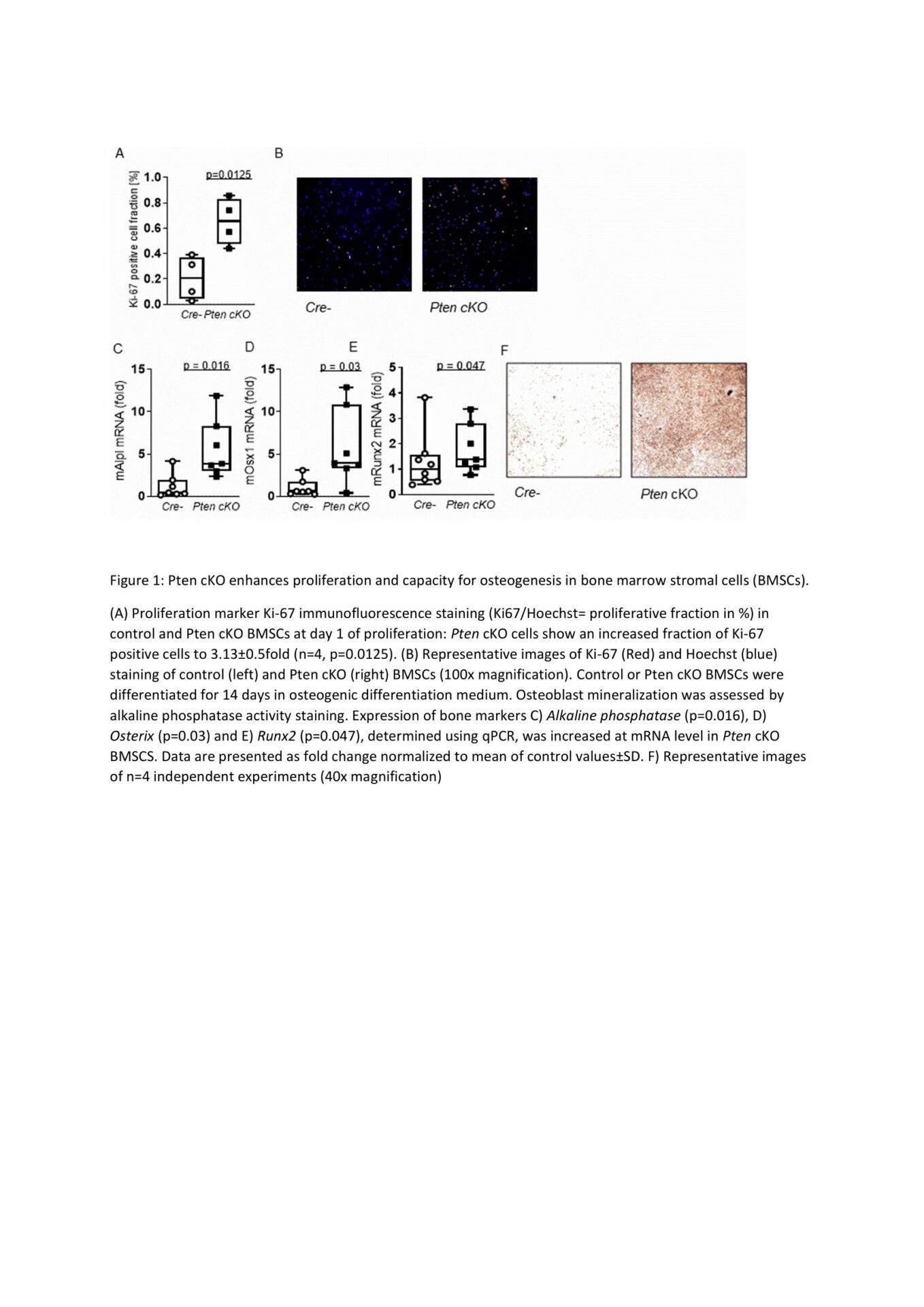
BMSCs from Pten cKO mice were functionally different from control BMSCs. Osteogenic marker Runt-related transcription factor 2(Runx2) was increased 5.4fold in BMSCs from Pten cKO mice, while Pten protein was lowered to 0.7±0.1fold (normalized to α-Tubulin, p=0.003) and AKT(S473) phosphorylation was increased to 11.2±1.1fold (normalized to total AKT, p=0.03) compared to control BMSCs (n = 3 per group). We detected a higher trabecular bone volume/total volume (males: 1.8±0.3fold, p=0.07) and higher trabecular bone mineral density (males: 1.7±0.2fold, p=0.08) in Pten cKO bones of both sexes, while cortical thickness was also increased (males: 1.2±0.06fold, p=0.04, n=6 per group). Biomechanical analysis revealed a significantly higher maximum force (3.7±0.6fold, p=0.0003) and increased elastic modulus (2.8±0.5fold, p=0.009, n=6 per group) of Pten cKO femora. Pten cKO bones from male mice had a higher number of osteoblasts per bone perimeter (1.9±0.3fold, p=0.004, n=4 controls, n=6 Pten cKO). Bone turnover markers P1NP and CTX were significantly increased both in Pten cKO male and female mice. Increased proliferation of isolated Pten cKO BMSCs was detected (males: p=0.0125, n=4 per group). Osteogenic differentiation capacity was significantly enhanced in BMSCs from both male and female Pten cKO mice as shown by Alkaline phosphatase staining and higher expression of Alkaline phosphatase (n=7, p=0.016), transcription factor Osterix (n=7, p=0.03) and Runx2 (n=8 controls, n=7 Pten cKO, p=0.047) (Figure 1), while adipogenic differentiation was not altered.
Pten knockout in osteoprogenitor cells increases stability and elasticity of mouse long bones and leads to increased proliferation and osteogenic differentiation of bone marrow stromal cells in vitro.