Background: Maximising the muscle anabolic response to dietary protein is crucial for muscle mass maintenance in older individuals, due to the presence of anabolic resistance contributing to sarcopenia (1). Within dietary protein, leucine is thought to be the most potent amino acid (AA) for stimulating muscle protein synthesis (MPS; 2, 3). Supplementing leucine-enriched essential AA and/or whey protein (WP) has previously been shown to be as anabolic as larger doses of protein (4), thus may negate the need for large protein meals, especially in clinical settings. Beta-lactoglobulin (BLG) is the most abundant WP in bovine milk and has a high content of branched chain amino acids (BCAA), especially leucine (5). We, therefore, investigated the impact of BLG versus WP isolate (WPI), at a suboptimal protein dose, on blood aminoacidemia and MPS in healthy older males.
Methods: In this double-blind cross-over trial, 10 healthy older males (69 ± 1 years, 181 ± 2 cm, 92 ± 5 kg) were randomised to receive either i) BLG (1.5 g leucine) or ii) WPI (1 g leucine), with supplements matched for protein content (10 g). A primed, constant intravenous infusion of [1, 2 13C2] leucine was used to determine MPS at baseline and following feeding. Muscle biopsies were taken at 1.5 h after commencement of stable isotope tracer infusion (i.e., 0 h), immediately before a bolus feed (3 h; i.e., BLG or WPI) and 3 h post-feed (6 h). Serial arterialised blood samples were obtained to quantify plasma AA concentrations. Data are presented as mean ± SEM. MPS data were analysed using a two-way ANOVA (supplement x time) with a Šídák correction. Plasma AA data were analysed using a mixed-effects analysis (supplement x time) with a Šídák correction. All data analysis was performed using GraphPad Prism (GraphPad Software Inc, San Diego, CA). The alpha level of significance was set at p < 0.05.
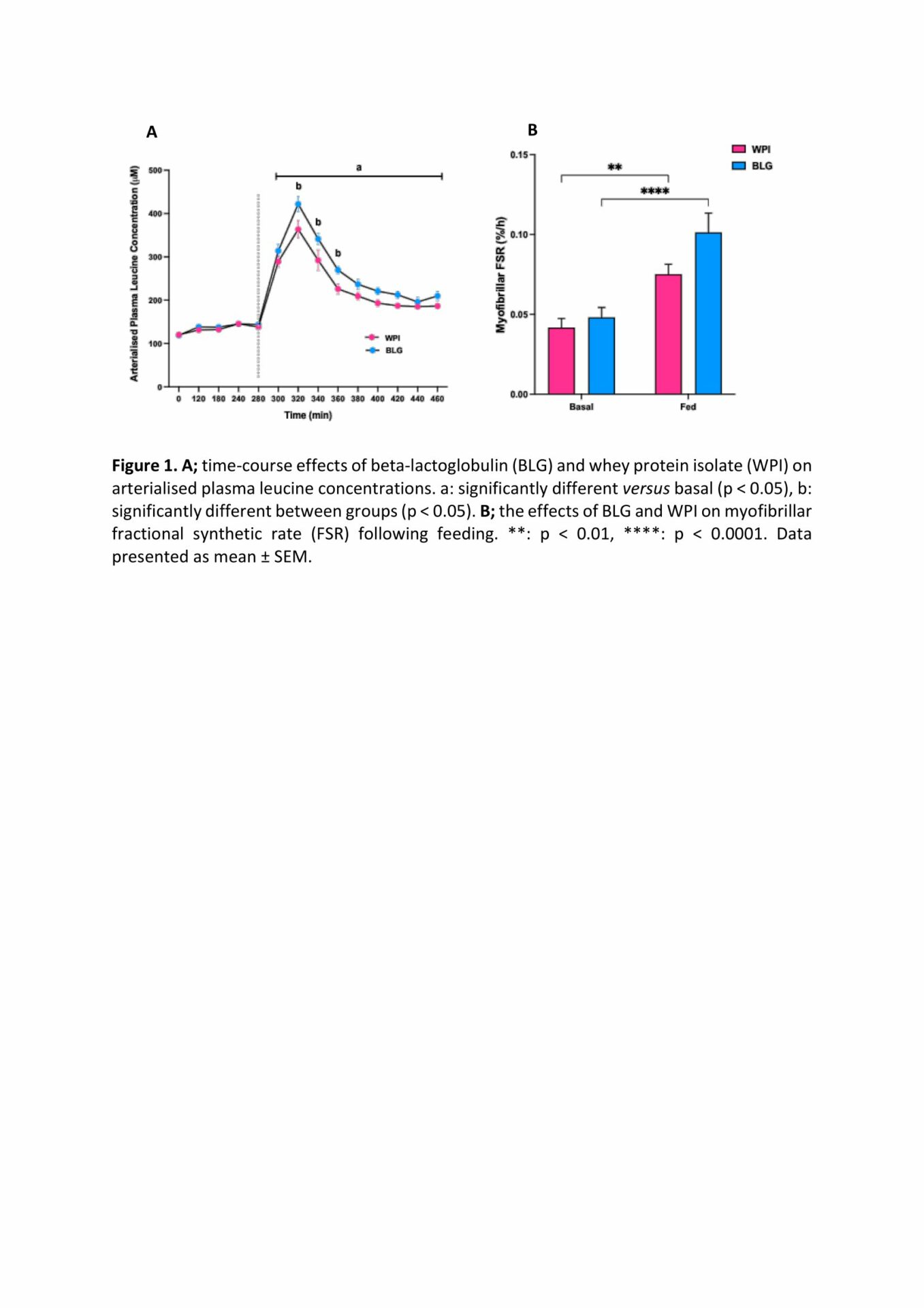
Results: Blood plasma BCAA, leucine, isoleucine and valine concentrations significantly increased following feeding in response to BLG and WPI before returning to baseline, except plasma leucine which remained elevated in both groups. Peak plasma leucine concentrations were significantly greater following BLG (BLG: 422 ± 17 µM versus WPI: 364 ± 20 µM, p = 0.0013). Conversely, peak isoleucine concentrations were significantly greater following WPI (269 ± 34 µM versus BLG: 165 ± 14 µM, p < 0.0001). Myofibrillar MPS increased significantly following feeding (BLG; fasted: 0.048 ± 0.006 %/h, fed: 0.101 ± 0.012 %/h, p < 0.0001 versus WPI; fasted: 0.042 ± 0.006 %/h, fed: 0.075 ± 0.006 %/h, p = 0.0032), with a strong trend to BLG stimulating greater MPS than WPI post-feed (p = 0.052).
Conclusion: BLG exhibited greater peak leucinaemia compared to WPI, with both BLG and WPI significantly stimulating MPS following feeding. There was a strong trend to BLG stimulating greater MPS compared to WPI which may be physiologically significant, especially in larger trials. We conclude BLG containing a suboptimal dose of protein leads to an anabolic response to feeding in healthy older men.