Cerebral hypoxia is a feature for several neurological diseases. The low concentration of oxygen and existing inflammation in the brain in these diseases forms a vicious circle that damages brain cells and inhibits the brain regeneration. Neuroinflammation is thought to potentially play both a positive and negative role in hypoxic injury since, depending on severity and duration of the pro-inflammatory stimuli, with prolonged stimulation being associated with activation of cell death pathways and the promotion of damaging oedema and systemic immune invasion. As such, better understanding the ways in which hypoxic stimulation alters the inflammatory activity of microglial cells (the specialised form of immune cells unique to the CNS) is vital to understanding and exploiting endogenous neuroinflammation during hypoxic injury.
To investigate the role of hypoxia in neuroinflammation we established a model of microglial inflammatory stimulation, using a dosage curve of Lipopolysaccharide (LPS) from 1ng/mL to 10,000ng/mL, which were applied to both mouse primary microglial cells and a microglial cell line — BV-2 cells in either normoxia (21% O2) and hypoxia (1% O2) oxygen exposures, for 24h. Responses were measured in terms of cell viability, cellular death, TNF-a production, and inflammatory gene expression, as well as phagocytosis through immunofluorescence imaging. Further exploration of the role of established cellular hypoxic response pathways, specifically the role of the Hypoxia Inducible Factors (HIF) 1 and 2, was then conducted by observing how the established cellular responses altered by co-exposure of the cells to 100uM of a clinically approved 100uM PHD2 inhibitor, namely FG4592 (Roxadustat).
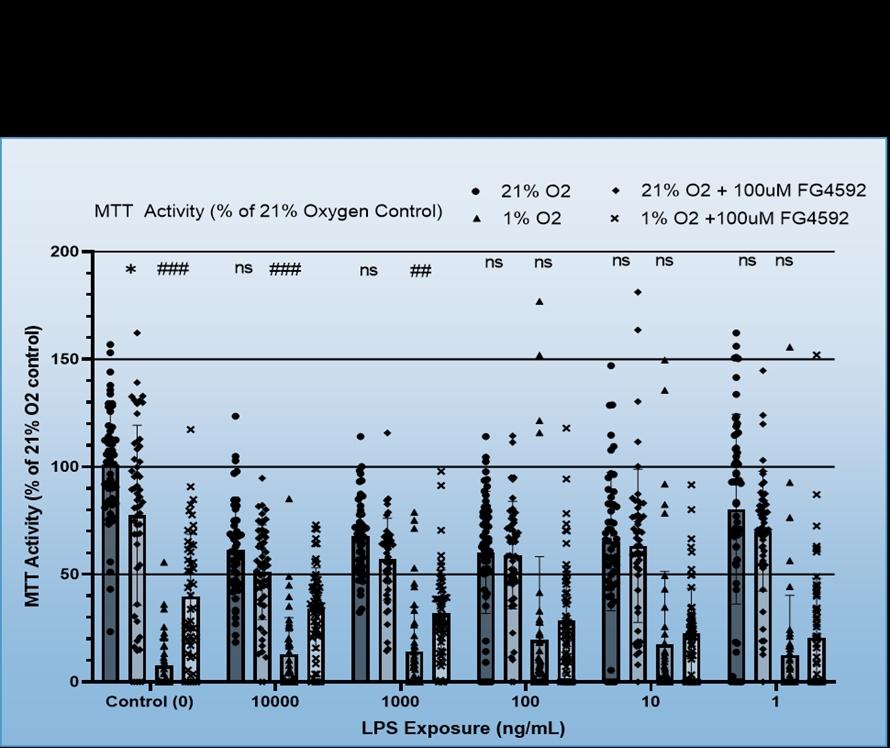
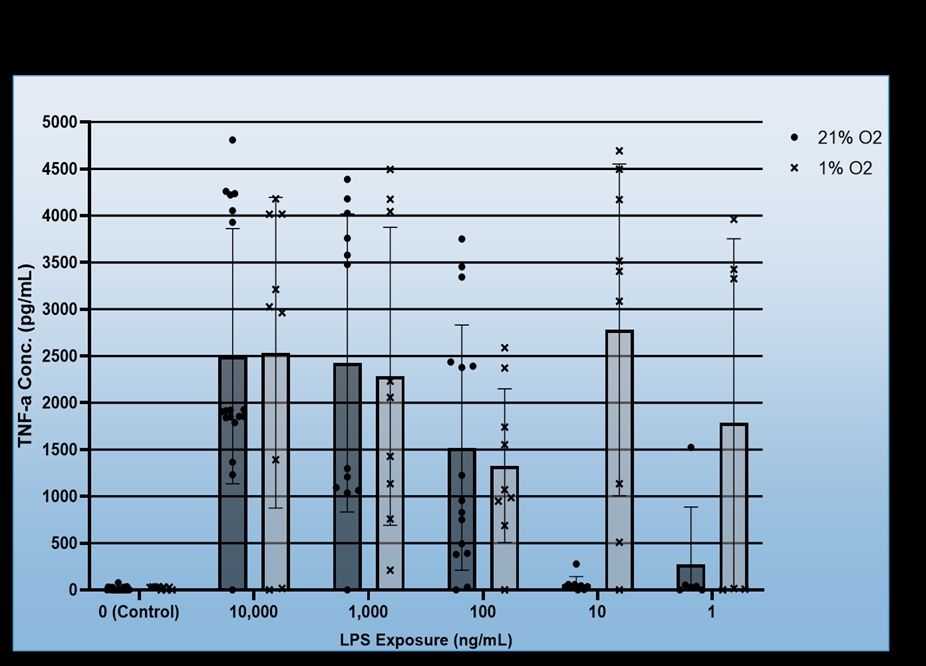
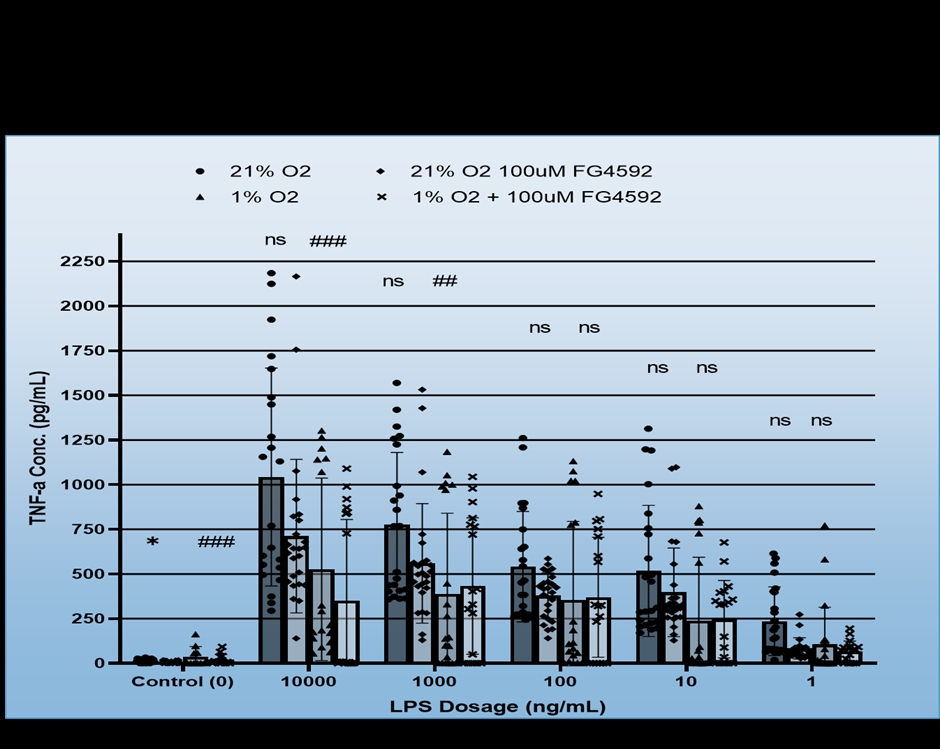
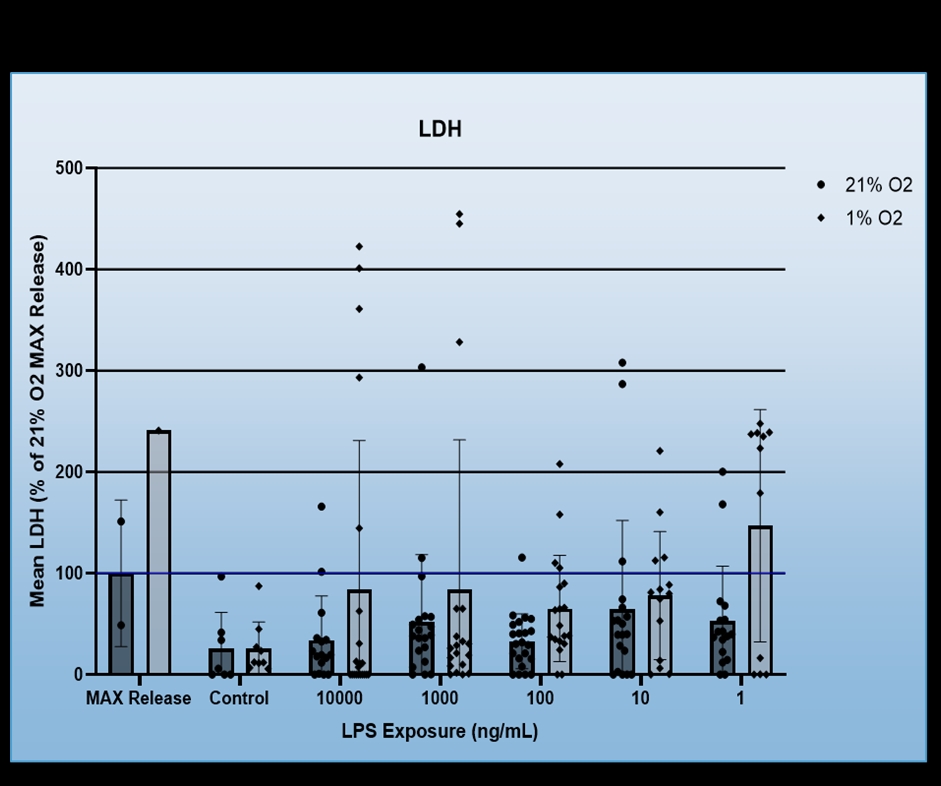
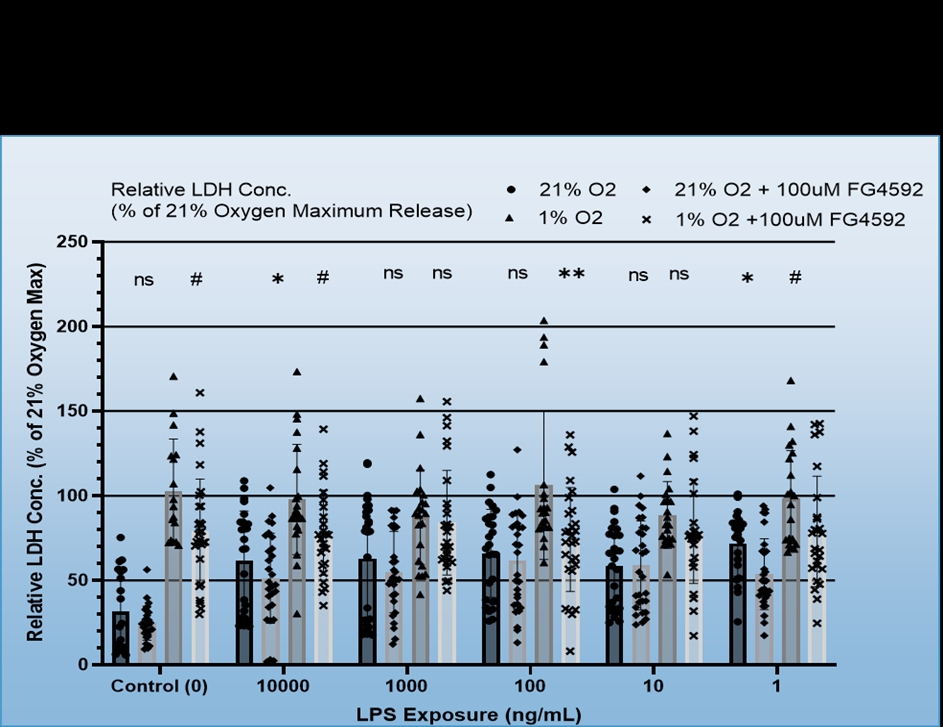
We found that BV-2 cells generally showed decreased inflammatory signalling, i.e. TNF-a production in response to hypoxia regardless of LPS dosage, but also that hypoxia did not induce inflammatory signalling in the absence of the LPS (Figure 1). No consistent effect for FG4592 on TNF-a production was identified during hypoxia, however, it did both non-significantly reduce TNF-a expression for all LPS dosages in normoxia and ameliorate hypoxia induced cell death (Figure 2). Mouse Primary microglia displayed a more varied response. Hypoxia generated an apparent increase in cell death provided LPS stimulation was present (Figure 3), unlike in BV-2 cells where hypoxic death was not dependent on LPS stimulation, and it only appeared to promote existing inflammatory signalling at low levels of LPS stimulation (10ng/mL + 1ng/mL), which may suggest hypoxia limits the inflammatory activity of already stimulated/activated microglia but acts as an inflammatory promotor itself when such activation would otherwise be limited (Figure 4). Our phagocytosis assay results were inconclusive.
Our findings suggest that hypoxia limits inflammatory activity by BV-2 cells but may promote it in primary microglia when total inflammatory stimulation is limited. Additionally, inflammatory stimulation seemingly makes primary microglia, though not BV-2s, more susceptible to hypoxic cell death. FG4592 was effective in ameliorating hypoxic cell death and metabolism loss, seemingly through inflammation independent mechanisms in BV-2 cells (Figure 5.)