Introduction
Sympathetic activation is a hallmark of cardiac disease, contributing to disease progression and triggering ventricular arrythmias (VA)1. While implantable cardioverted defibrillators (ICD) improve mortality by terminating VAs, many patients experience recurrent ICD therapies, leading to worse outcomes and quality of life2. Cardiac sympathetic denervation (CSD) through surgical removal of the stellate ganglia has emerged as an effective treatment for refractory VAs3 but carries a complication rate of up to 50%3,4. Direct cardiac radiotherapy to treat refractory VA has also been shown to be both safe and effective5, but whether radiotherapy can be targeted at the stellate ganglia is unknown. We therefore hypothesised that high precision image guided radiotherapy can be used to target the stellate ganglia to achieve CSD non-invasively leading to fewer complications while still maintaining efficacy.
Methods
RADIO-STAR (ISRCTN 49861434, REC/SC/0005) is a phase 1 clinical trial to determine the feasibility and safety of non-invasive CSD achieved through image guided radiotherapy to the stellate ganglia. 13 patients with structural heart disease who experience recurrent ICD therapy during the preceding 6 months despite optimum medical therapy are invited to undergo image guided radiotherapy bilaterally to the lower half of the stellate ganglia and T1-2 sympathetic chain. A baseline 1.5T MRI of the stellate ganglia is used to determine feasibility of delivering radiotherapy to the stellate ganglia within dose constrains of nearby organs at risk and ICD devices. Radiotherapy is then delivered over 3 fractions on alternate days with total dose escalating from 24 to 33 Gy according to a dose escalation protocol. Participants are followed up for 6 months including continuous ICD device monitoring and repeat stellate ganglion MRI. Safety (adjourned by an independent safety committee) is the primary outcome, and the study is powered (β = 80%, α = 0.05) to detect a serious adverse event rate of 34%. Secondary outcome measures to evaluate efficacy include radiotherapy induced changes in circulating catecholamine and neuropeptide-Y levels, ICD therapy burden, heart rate variability (HRV) and structural changes in the stellate ganglia on MRI imaging.
Results
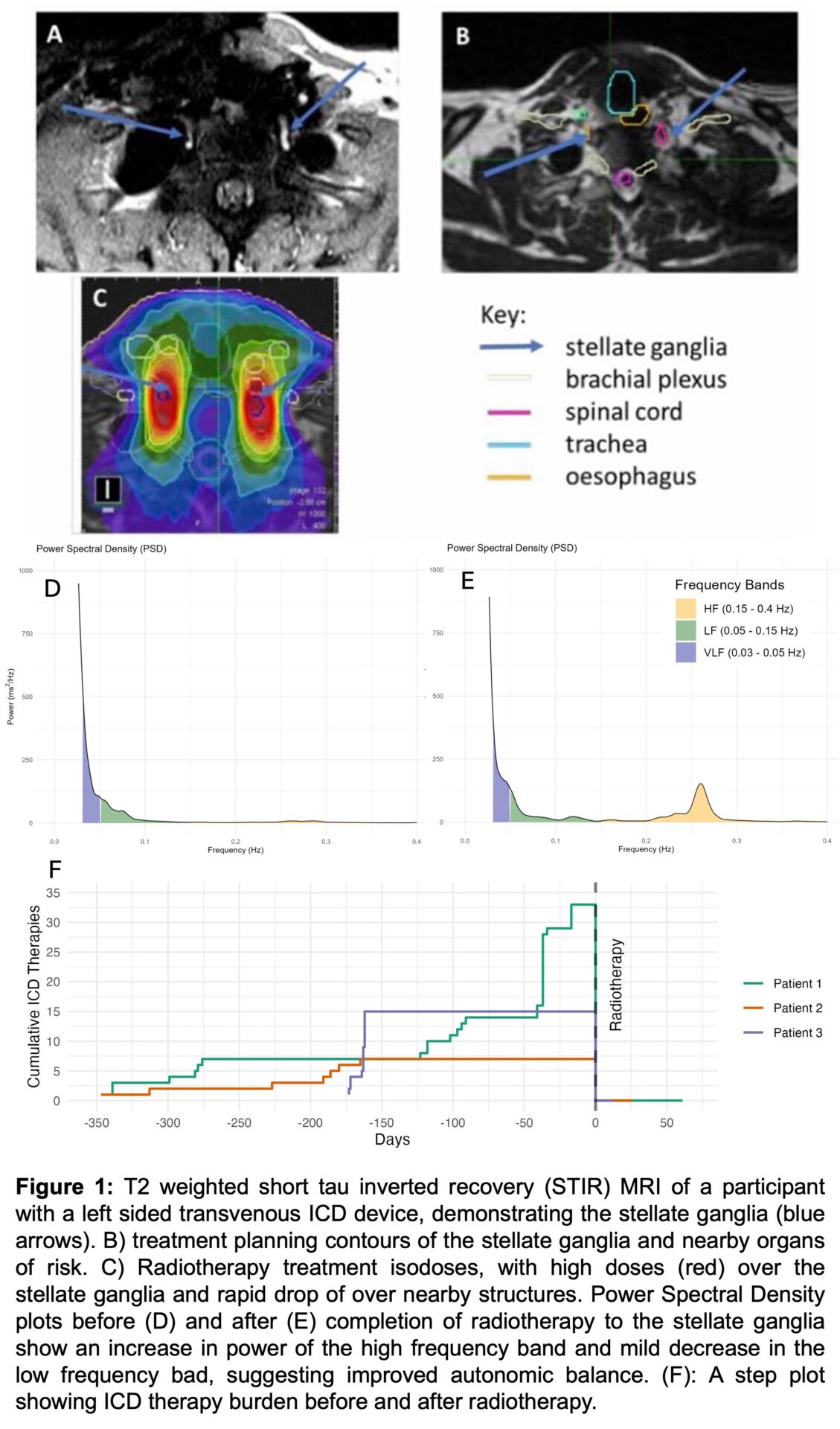
We have developed MRI protocols that allow clear visualisation of the stellate ganglia, while limiting ICD related image artefacts (Figure 1A-C). In all 5 patients that so far have undergone baseline MRI it was possible to clearly visualise the stellate ganglia and plan for radiotherapy within dose constraints of nearby organs at risk. To date, three patients have completed radiotherapy treatment (24 Gy), without serious adverse events. All patients remain arrhythmia free following treatment, with improved autonomic balance on HRV measurements (Figure 1D-F).
Conclusion
We have developed a protocol for targeting the stellate ganglia with radiotherapy to achieve non-invasive CSD. Preliminary findings indicate that this approach is feasible and appears safe; however, further patient recruitment is needed to confirm safety and evaluate efficacy.