Introduction: Obesity affects 1 in 8 people globally and has been independently associated with arrhythmia incidence, including atrial fibrillation (AF). Standard anti-arrhythmic therapies have shown poorer response rates in obese patients. Weight loss has been shown to improve AF outcomes. However, the mechanisms underlying obesity-mediated pro-arrhythmia and the therapeutic effects of weight loss remain unclear. This study aimed to determine whether weight loss reverses obesity-induced left atrial pro-arrhythmic remodelling in mice fed a high-fat diet (HFD).
Methods: Male and female CD1 mice (5-8 weeks old) were assigned to three groups: 1) control (normal chow and water for 20 weeks), 2) HFD (60 kcal% fat) with 15% fructose water for 20 weeks, or 3) weight loss (HFD and fructose water for 14 weeks, followed by normal chow and water for 6 weeks). In vivo echocardiography, ECG, ex vivo left atrial optical mapping and O-link plasma proteomics were performed at 19 to 20 weeks.
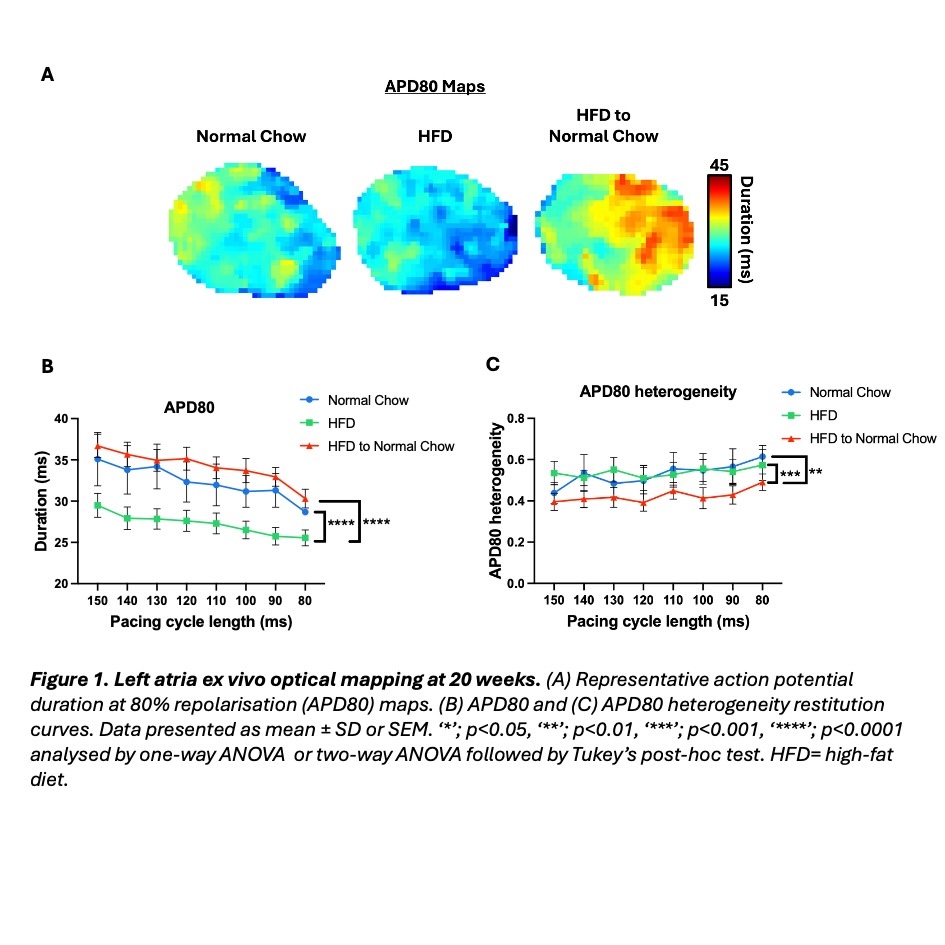
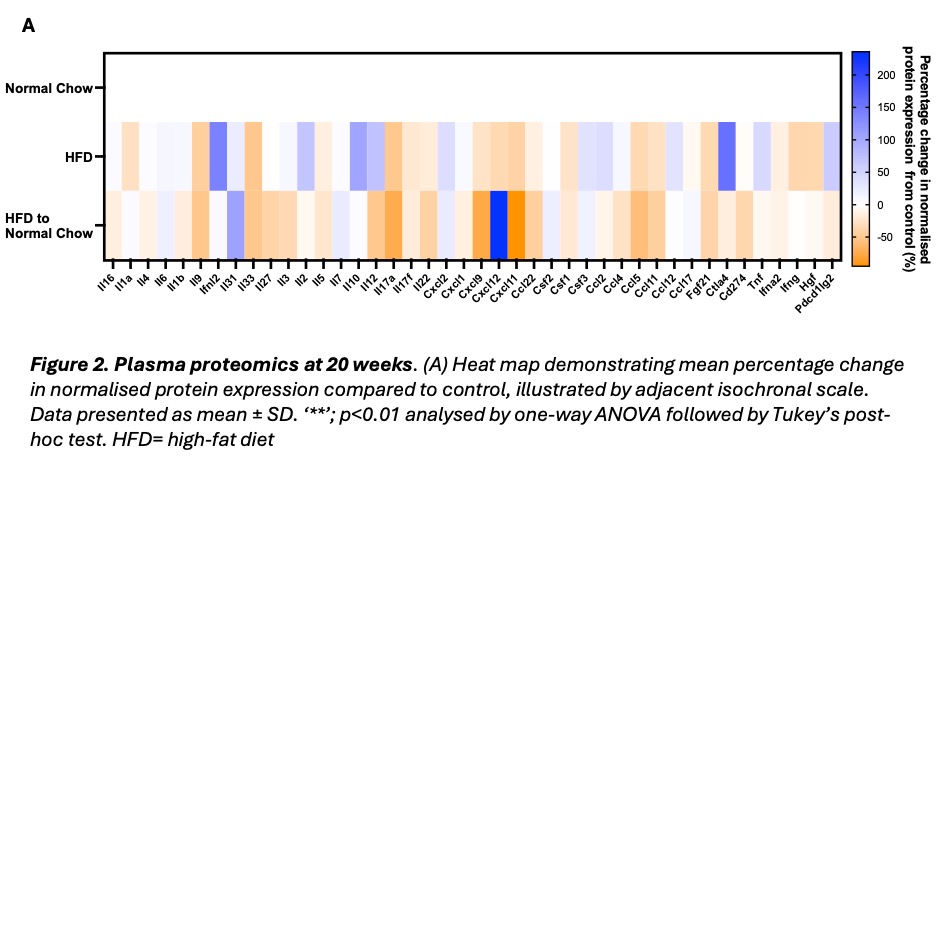
Results: HFD mice demonstrated significantly higher body weight (61.9 ±7.24g versus 40.8 ± 5.75g, p<0.0001) and blood glucose (18.3 ± 3.19 mMol/L versus 14.9 ± 2.34 mMol/L, p<0.05) versus control. The weight loss group achieved significant body weight reduction compared to HFD (45.5 ± 9.78g versus 61.9 ±7.24g, p<0.01). Systolic, diastolic and myocardial strain parameters were unchanged across all groups. HFD mice also showed similar heart chamber dimensions to control. ECG characteristics were also similar between groups, except for reduced R wave amplitude in HFD and weight loss mice versus controls (control: 0.601± 0.0591mV, HFD: 0.416±0.0457mV (p=0.0644), weight loss: 0.379 ±0.0495mV (p<0.05)). Optical mapping revealed shortened left atrial action potential duration at 30, 50 and 80% (APD30, APD50, APD80) repolarisation for HFD mice, compared to control (Figure 1, A-B). Weight loss attenuated APD prolongation and reduced APD80 spatial heterogeneity (Figure 1, A-C). Left atrial effective refractory period (ERP) showed a similar trend, although non-significant, where HFD mice displayed a shorter ERP than control (42.4 ± 6.99 ms vs 57.6 ± 8.76 ms). Weight loss partially recovered the ERP (46.0 ± 11.6 ms). Plasma proteomics demonstrated no significant alteration in overall systemic inflammation between groups (Figure 2, A). However, CCL11 and CCL5, markers linked to myocardial fibrosis and heart failure, were significantly reduced in weight loss mice compared to control.
Conclusions:
20-week HFD induced significant left atrial pro-arrhythmic remodelling, with preserved systolic and diastolic function. Notably, HFD-associated pro-arrhythmic changes occurred in the absence of systemic inflammation. Weight loss ameliorated HFD-induced atrial electrical remodelling, further supporting weight management as a strategy for mitigating obesity-related AF risk.