Background:
Atrial fibrillation (AF), the most prevalent sustained arrhythmia, is strongly associated with genetic variants near the PITX2 locus on chromosome 4q25. Recent studies suggest an inverse regulatory relationship between PITX2 and bone morphogenetic protein 10 (BMP10), a developmental factor increasingly recognized as a potential circulating biomarker for AF. However, mechanistic data linking PITX2 regulation to BMP10 release levels and its impact on cardiac electrophysiology remain limited.
Purpose:
We aimed to quantify cardiac BMP10 mRNA and protein in adult human heart chambers and determine baseline cardiac BMP10 release from isolated primary human cardiomyocytes. Further we set to quantify Pitx2 expression across cardiac chambers of wildtype (WT), heterozygous (HET), and homozygous (HOM) mice with a Pitx2 enhancer deletion and detect chamber-specific Bmp10 protein release in those groups. Finally we wanted to characterize electrophysiological differences in left atria (LA) of mice with a Pitx2 enhancer deletion, including their response to sodium channel blockade by flecainide.
Methods:
We used discarded atrial tissues from cardiac surgery—primarily small samples from appendages of both atria—to quantify BMP10 gene and protein concentrations in the human heart. mRNA was quantified by RT-PCR. BMP10 protein concentrations in cardiac chamber lysates and culture supernatants of isolated primary human cardiomyocytes were quantified by ELISA. Murine Pitx2c expression was quantified via RT-PCR (n=6). LA, RA, and ventricular tissues from Pitx2 enhancer deletion mouse model were cultured ex vivo, to assess Bmp10 release in supernatant using ELISA (n=12). Electrophysiological properties of isolated murine LA (n=12) were evaluated via sharp microelectrode recordings at multiple pacing cycle lengths, at baseline and after 1 µM flecainide perfusion.
Results:
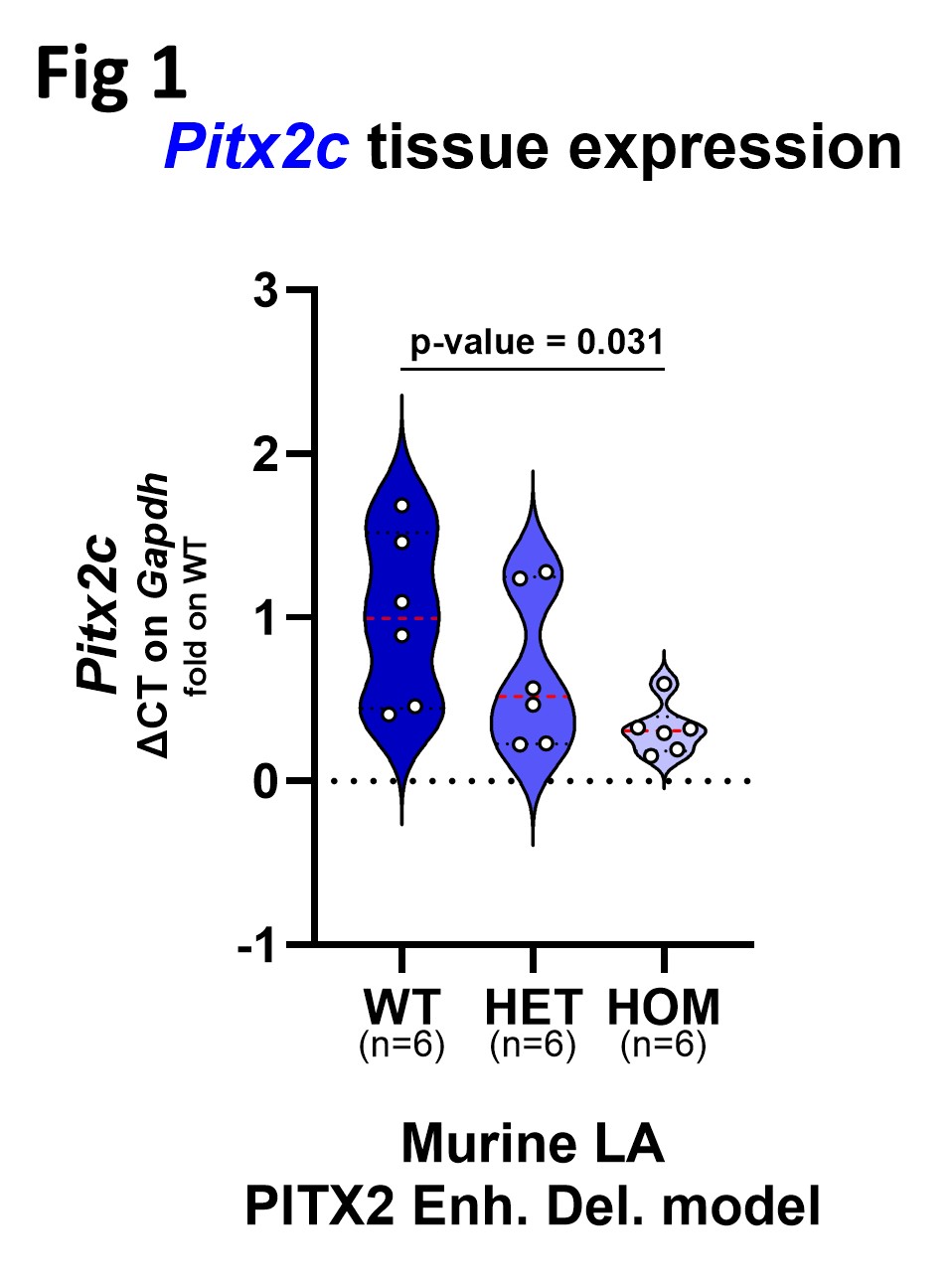
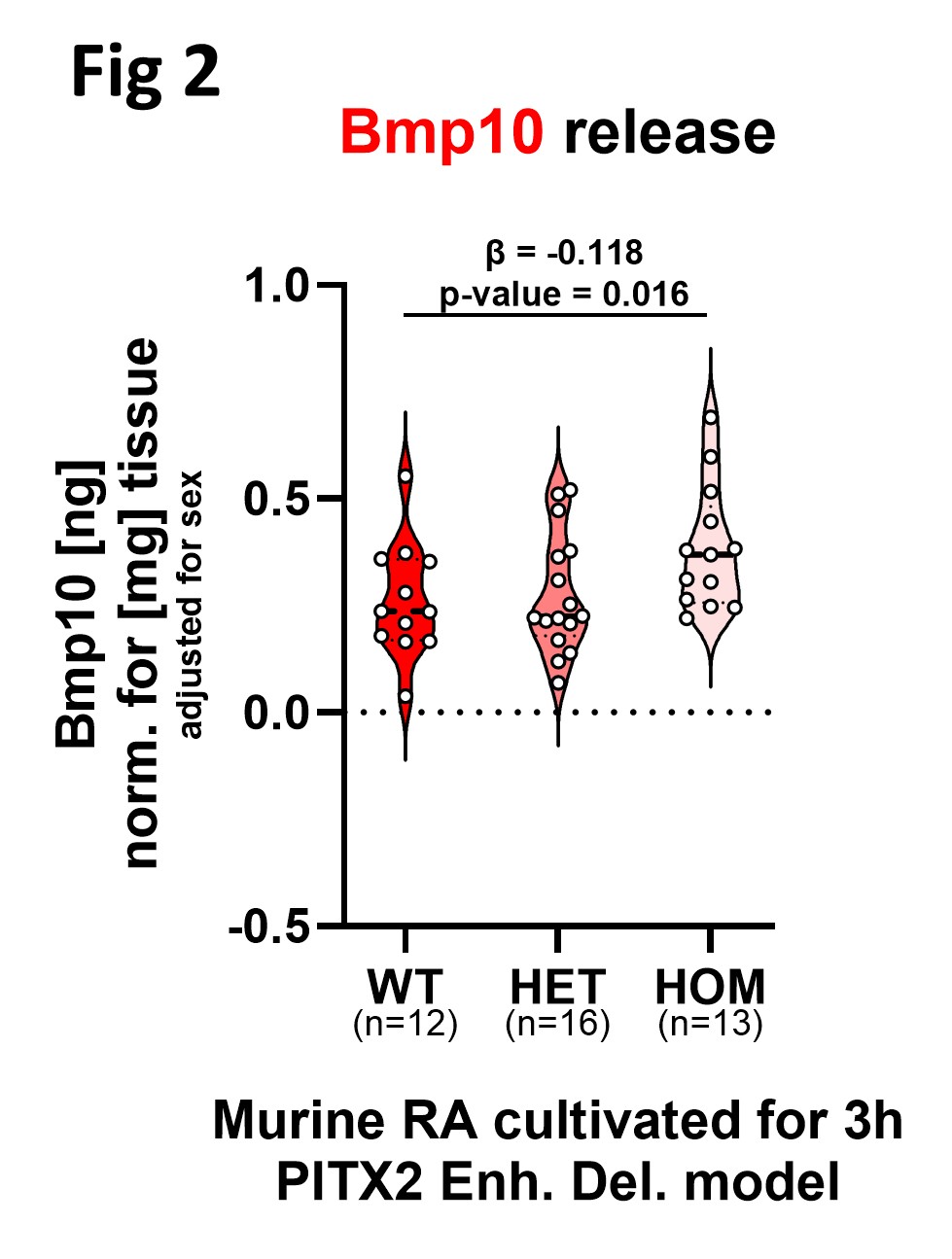
In human cardiac chambers RA tissue showed highest BMP10 expression on gene and protein level. Compared to LA protein expression BMP10 was ~240-fold higher in RA. Culture supernatants of primary human cardiomyocytes isolated from the RA had highest BMP10 amount compared to other chambers. LA Pitx2c expression was significantly reduced in mice with a homozygous Pitx2 enhancer deletion to 1/3 of WT levels (Fig 1). RA tissue released detectable Bmp10 ex vivo, with HOM RA showing the highest release (0.383 ± 0.04 ng/mg) (Fig 2). LA electrophysiology showed genotype-dependent changes in resting membrane potential, upstroke velocity (Vmax), and action potential duration (APD90).
Conclusions:
This study establishes the adult human and murine RA as the predominant cardiac source of BMP10 synthesis and release. Further it shows that reduced Pitx2 expression via enhancer deletion is associated with increased Bmp10 release and LA electrophysiological remodelling.
Novelty:
This study provides the first quantitative evidence of BMP10 in both -expression and release- in human and murine heart tissue and cardiomyocytes. We demonstrate that reduced Pitx2 expression in the LA is associated with increased Bmp10 release from the right atrium. In a mouse model with a Pitx2 enhancer deletion, we establish a link between the enhancer deletion, left atrial Pitx2 expression and altered electrophysiological properties. These findings support a functional link between Pitx2 and Bmp10 in atrial pathophysiology, relevant to atrial fibrillation and potential biomarkers of atrial fibrillation.