Physiologically, K+-Cl– cotransport (KCC) in human red blood cells (RBCs) contributes to regulatory volume decrease. It is also present at greater activity in sickle cells, where it mediates inappropriate cell shrinkage, thereby contributing to the pathophysiology of sickle cell disease. In vivo [H+] represents another important modulator of transport activity (Ellory et al. 1990). The H+ ion dependence is often described as bell-shaped, with maximum activity at ca pH 7.0, with inhibition at lower and higher pH values (e.g. Brugnara & Tosteson, 1987). The effect of pH values higher than about 8, however, has not been studied extensively.
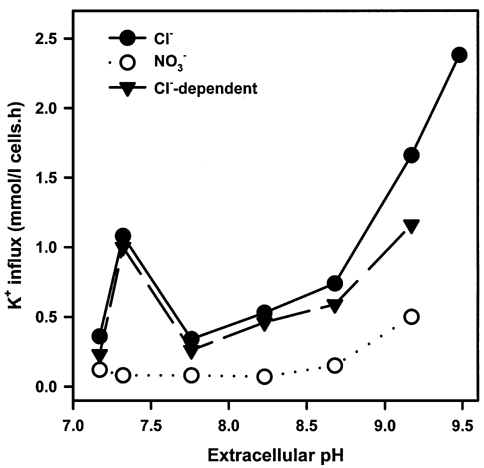
In this communication, we measured K+ influx into normal human RBCs, obtained with consent and ethical permission, at various external pH values. 86Rb+ was used as a K+ congener, in the presence of 500 mM urea. The methodology is described fully in previous publications (e.g. Gibson et al. 1998). The relationship between K+ influx and pH is shown in Figure 1. The normal bell-shaped relationship centred on ca pH 7.3 was present, but influx was also markedly increased at very alkaline pH values, from 8 to 9. At pH 9, K+ influx was 2.8 ± 0.4-fold (mean ± S.E.M., n = 8) higher than at pH 7. This component of transport was inhibited by substitution of NO3– for Cl– (e.g. by 68 %, pH 9) and by treatment with the protein phosphatase inhibitor calyculin A (100 nM, e.g. by 66 %, pH 9), implying that the greater part was via KCC.
These findings show a more complicated relationship between KCC activity and pH than is usually accepted. The transporter is markedly stimulated at alkaline pH values, possibly due to reduction in free intracellular [Mg2+] (following increased ionisation of organic phosphates at high pH), or via a decrease in [Mg2+-ATP], both acting via the regulatory phosphorylation cascade. Elucidating the mechanism of this effect is important for understanding normal regulation of KCC and may be relevant to its abnormal behaviour in sickle cells.
We thank Action Research and The Wellcome Trust for financial support.