Amino acid (AA) transport by System A is subject to regulation by a variety of physiological factors (McGivan & Pastor-Anglada, 1994). Since the System A Transporter isoforms (SAT1-3) were recently cloned (Christie et al. 2001), molecular mechanisms for such regulation are now open to investigation. Prolonged AA deprivation increases System A Vmax (adaptive upregulation); in L6 myotubes this occurs concomitant with increased SAT2 expression (Hyde et al. 2001). Previous reports indicate a role for microtubules in adaptive upregulation, proposing that their disruption prevents the translocation of newly synthesised transporters (Guma et al. 1992). Here we investigate cytoskeletal involvement in the adaptive regulation of SAT2 in L6 muscle cells following AA deprivation.
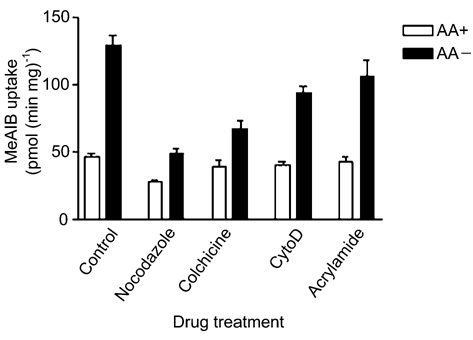
Incubation of L6 myotubes in Earl’s balanced salt solution (EBSS) for 4 h increased System A transport of methylaminoisobutyric acid (MeAIB) by 82.5 pmol min-1 mg-1, compared with amino acid supplemented EBSS (see Fig. 1). Incubating cells with nocodazole (33 µM) or colchicine (10 µM), disruptors of the microtubule network, reduced this increase to 10.3 and 27.5 pmol min-1 mg-1, respectively. Cytochalasin D (2 µM) and acrylamide (5 mM), which disrupt alternative cytoskeletal elements, had a lesser effect on this increase.
We investigated whether microtubule disruption suppressed adaptive upregulation by altering SAT2 expression. In total cellular membranes from AA-deprived L6 cells, the level of SAT2 was increased by 50 % compared with cells maintained in AA-containing media. This increase in SAT2 expression was inhibited by microtubule disruption. Furthermore, in subcellular membrane fractions of L6 cells a robust increase in intracellular SAT2 was observed upon AA deprivation. Nocodazole treatment reduced the level of SAT2 in this pool and prevented the increase following AA starvation.
These results imply that microtubules play a role in the cellular response to amino acid starvation. However, since microtubule disruption prevents SAT2 accumulation, the microtubules may play a role in regulating transporter expression, rather than in the delivery of newly synthesised transporters to the plasma membrane.
This work was supported by the BBSRC and SmithKline Beecham.