Neuronal depolarization leads to intracellular pH (pHi) shifts as a result of transmembrane fluxes of acid equivalents. In snail neurones depolarization beyond ~+20 mV leads to intracellular alkalinization due to the opening of proton channels. Smaller depolarizations lead to acid shifts as a result of Ca-H pump activity. The sizes of the pHi shifts depend upon the surface area to volume ratio of the intracellular region (pH microdomain: Schwiening & Willoughby, 2002) leading to pHi gradients. We have investigated the dynamics of the pH transients in the soma and axon using ratiometric measurements from the pH-sensitive dye HPTS in the absence and presence of mobile buffers.
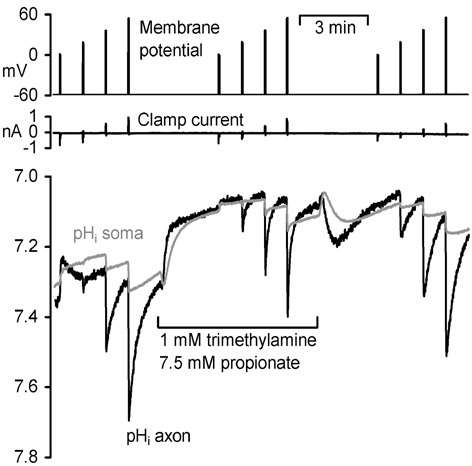
Neurones were isolated by enzymatic and mechanical treatment (Schwiening & Willoughby, 2002). A cell was patch-clamped and dialysed with 110 mM CsCl, 5 mM Hepes and 500 µM HPTS. Fluorescence (> 510 nm) was collected simultaneously from the soma and axon, by fibre optics, during illumination with alternating 458 nm and 380 nm light (Cairn, UK). This pH-sensitive ratio was calibrated in vitro. The neurone was held at -60 mV and depolarized at intervals before, during and after application of a mixture of buffers (Fig. 1).
The axonal alkaline shifts induced by depolarization to +60 mV recovered with a time constant (τ) of 18 ± 3 s (mean ± S.E.M.) compared with 68 ± 17 s in the cell body (n = 6). In the presence of the weak acid and base mix τ fell by 54 ± 4 % in the axon (n = 6) and by 66 ± 8 % in the cell body (n = 5). The buffer, however, only reduced the peak pHi shift by 20 ± 6 % in the axon and 23 ± 5 % in the cell body (n = 6). This is smaller than the ~50 % fall predicted from the open system buffering power (13 mM) produced by this buffer mix at pHi 7.1.
In conclusion, these ratiometric data are in good agreement with previously reported pH gradients (Schwiening & Willoughby, 2002) measured using single wavelength excitation. Furthermore membrane permeable hydrogen ion buffers may not reduce the peak size of the pH gradient as predicted simply by their open system buffering power, but they can cause the pHi gradients to collapse more rapidly by increasing intracellular proton mobility.
Antonios Pantazis was supported by a Wellcome Trust Vacation Scholarship; we also thank the MRC for financial support.