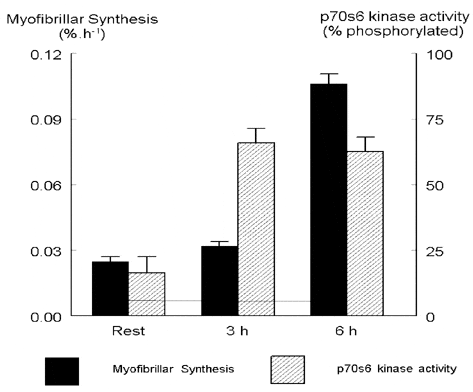
We aimed to determine if there was any difference in the extent and time course of stimulation of myofibrillar protein synthesis and the activity of p70S6k between contractile activity with muscle shortening or stretching. The study was approved by the Tayside Ethics Committee under the rules of the Helsinki agreement. We studied three healthy young men (21.3 ± 0.6 years, BMI 24.3 ± 1.15, means ± S.E.M.) over 6 h. The subjects, carrying 25 % body weight, stepped up with one leg onto a knee-high box and stepped down with the other at ~1 step s-1, for 6 min. After 2 min rest, they performed two further 3 min bouts. Quadriceps muscle biopsies were taken (using 1 % lignocaine anaesthesia) by the conchotrome technique, before and 3 and 6 h after exercise. Subjects were given seven drinks (each containing 6 g glucose and 2 g of mixed essential amino acids) over the 2 h preceding each biopsy. To determine protein synthesis we infused [1-13C] leucine (prime 1.0 mg kg-1 h-1; 0.8 mg kg-1 h-1) and measured its incorporation into the myofibrillar fraction (separated from needle biopsy samples of quadriceps by salt extraction, centrifugation and hydrolysis) by gas chromatography-combustion mass spectrometry. Phosphorylation of p70S6k (which correlates with its activity) was quantified on Western blots using an antibody recognizing all forms of p70S6k.
There were only minor differences in the responses of the muscle to the two types of exercise, with the ‘eccentric’ exercise causing slightly bigger increases in the values measured and they have therefore been averaged. Exercise resulted in a significant stimulation of p70S6k activity at both 3 and 6 h post-exercise (P < 0.01, paired t test). Myofibrillar protein synthesis increased by only ~25 % at 3 h after exercise but by ~4-fold at 6 h post-exercise (P < 0.01, paired t test). There was a lag between p70S6k activation and myofibrillar protein synthesis, as might be expected from their relative proximal and distal positions in the anabolic pathway. The extent of the rise in myofibrillar turnover is much greater than normally seen for mixed muscle protein.
This work was supported by the Medical Research Council and The Wellcome Trust.