The main exchange barrier of the placenta is the syncytiotrophoblast (syn). This is a multinucleated epithelium, which is formed throughout gestation by fusion and differentiation of underlying mononucleate cytotrophoblast cells (cyts). Cyts can be isolated from the human term placenta and when maintained in primary culture they undergo a process of differentiation, which is similar to that occurring in vivo. Differentiation of cyts is associated with an increase in human chorionic gonadotrophin (hCG) secretion; thus hCG production provides a biochemical marker of cell multinucleation (Kliman et al. 1986). Despite the advantages of this culture model for study of placental transport mechanisms, cyts have not previously been isolated from placentas of diabetic women. The aim of this study was to characterise and compare the morphological and biochemical differentiation of cyts isolated from placentas of non-diabetic women (C) and women with pre-existing insulin-dependent diabetes (IDDM) and pregnancy-induced glucose intolerance (IGT).
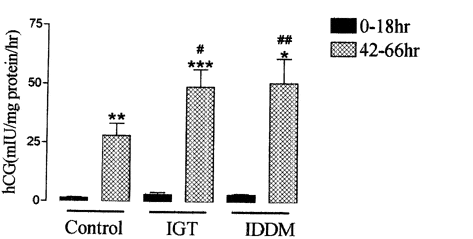
Cyts were isolated from placentas collected at 36-41 weeks gestation by digestion with trypsin and DNase, followed by purification using a discontinuous Percoll density gradient. Cyts were maintained in culture for up to 72 h. Culture medium was collected at 0-42 h and 42-66 h of culture. Production of hCG was measured by radioimmunoassay. Morphological differentiation of cyts was examined using immunofluorescence. Cells were fixed with methanol at 18 or 66 h in culture; for morphological evaluation cells were then stained with anti-desmosomal antibodies to reveal cell boundaries and anti-nuclear antibodies to reveal nuclei. To identify cells of epithelial origin, 66 h fixed cells were stained with anti-cytokeratin antibody; positive staining was quantified by counting number of nuclei in the field. The process of morphological differentiation was similar in cyts isolated from placentas of C, IDDM and IGT women. Following 18 h in culture cells were mainly mononucleate; by 66 h of culture the cells were predominantly multinucleated. The majority (▓ge│ 95 %) of cells isolated from C, IDDM, IGT placentas showed positive cytokeratin, indicating cells were of epithelial origin. hCG secretion by cyts increased significantly between 18 and 66 h (15- to 19-fold increase: C, n = 7; IDDM, n = 6; IGT, n = 10), whilst cells from placentas of diabetic women and non-diabetic women show a similar pattern of biochemical differentiation between 18 and 66 h of culture; hCG secretion by cyts isolated from both diabetic groups was significantly higher than that from C (Fig. 1, P < 0.05 IGT and P < 0.01 IDDM).
We conclude that cyts can be isolated from placentas of diabetic women. When maintained in culture these cells undergo a process of morphological and biochemical differentiation similar to that occurring in cyts isolated from non-diabetic women. The enhanced secretion of hCG in cells isolated from diabetic women compared with non-diabetic women may suggest differences in regulation of hCG secretion.
This work was funded by NHS Executive Trent Authority. Ethics committee approval and informed consent were obtained.
 View larger image [new window] |
| Figure 1. hCG secretion by isolated cyts cells. Means ± S.E.M. *P < 0.01, **P < 0.001, ***P < 0.0001 vs. 0-18 h, ANOVA + paired t test; #P < 0.05, ##P < 0.01 vs. 42-66 h, ANOVA + unpaired t test + Bonferroni. |