Interleukin-1β (IL-1β) is a primary mediator of immune responses to injury and infection, but the mechanism of its cellular release is unknown. IL-1β is synthesised as an inactive precursor that lacks a signal sequence, and is cleaved and released by caspase-1. ATP induces the processing and release of IL-1 (α and β) via activation of the P2X7 receptor. Experiments in primary murine macrophages derived from peritoneum (all animals were killed according to UK legislation) suggested a role for Ca2+ in the mechanism of IL-1β secretion in response to ATP.
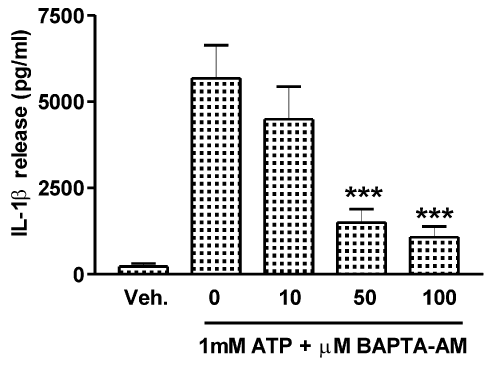
The calcium dependence of IL-1β release in response to ATP is shown in Fig. 1. Subsequent experiments were performed to identify the source of calcium, which was shown to be thapsigargin (TG)-sensitive ER stores.
However, release of Ca2+ from intracellular stores alone is not sufficient to trigger mature IL-1β release. For example, agents capable of inducing [Ca2+]i transients such as TG, 100 mM ATP, and the purely metabotropic stimulus PAF (platelet activating factor), failed to induce IL-1β release. In addition, treatment of the cells with ionomycin correlated with a high level of cell death, which was accompanied by the release of only pro-IL-1β. The use of the K+ ionophore nigericin, identified K+ efflux as the factor required in addition to elevated [Ca2+]i to promote the release of processed IL-1β.
In conclusion, we have demonstrated that ATP-induced IL-1β processing and release from murine peritoneal macrophages is dependent upon the release of Ca2+ from TG-sensitive stores. In addition, we demonstrate that this is necessary, but not sufficient to promote IL-1β release, as it also depends on K+ efflux.
We are grateful to the MRC and BBSRC for supporting this work.
All procedures accord with current UK legislation.