Polarised Ca2+ signals that originate at and spread from the apical pole have been shown to occur in acinar cells from lacrimal, parotid, submandibular and pancreatic glands. However, Ca2+ signals that are restricted to the apical pole of the cell have been previously demonstrated only in pancreatic acinar cells where the primary function of the Ca2+ signal is to regulate exocytosis. We have investigated whether local Ca2+ signals also occur in submandibular acinar cells where the primary function of the Ca2+ signal is to drive fluid and electrolyte secretion, a process that requires simultaneous activation of a basolateral K+ channel and an apical Cl– channel.
Acinar cells were isolated from the submandibular glands of humanely killed male CD-1 mice by collagenase digestion. For each experiment, cells were allowed to settle onto a poly-L-lysine-coated coverslip that formed the base of the perfusion chamber and placed on the stage of an inverted microscope. Patch-clamp whole-cell and microfluorimetry experiments were performed using cells loaded with fura-2 either by pre-incubation with fura-2 AM or via the patch pipette to measure simultaneously changes in [Ca2+]i and K+ and Cl– channel activity.
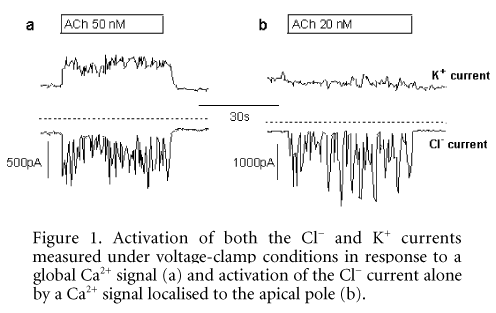
We show that the response to an intermediate (20Ð500 nM) concentration of ACh occurred as a series of transient increases in [Ca2+]i that originated at the apical pole of the cell and then spread as a Ca2+ wave to the basolateral pole. Each transient increase in [Ca2+]i caused activation of first the Cl– conductance and then the K+ conductance (Fig. 1a), thus confirming the distribution of these channels to the apical and basolateral poles, respectively, and demonstrating that this type of response would easily support fluid secretion. The response to a low (5Ð20 nM) concentration of ACh also comprised a series of transient elevations of [Ca2+]i, although these Ca2+ signals did not spread from the apical pole of the cell. Furthermore, only the Cl– and not the K+ conductance responded to local elevation of [Ca2+]i (Fig. 1b). This mode of activation might appear less effective in driving fluid secretion because the membrane depolarisation that results from Ca2+ activation of the Cl– conductance is not counterbalanced by Ca2+ activation of the K+ conductance. However, the effects of membrane depolarisation in these cells are self limiting because the K+ conductance is voltage as well as Ca2+ activated, and therefore the K+ conductance will increase even in the absence of Ca2+ activation.
Our data indicate that submandibular acinar cells are capable of local Ca2+ signalling and, by introducing a role for voltage activation of the K+ conductance into the model, that these Ca2+ signals could support fluid secretion.
This work was supported by a Wellcome Trust Prize Studentship to A.R.H.
All procedures accord with current UK legislation.