University of Central Lancashire / University of Liverpool (2002) J Physiol 543P,
S177
Communications: Characterisation of the intestinal peptide transporter of the Antarctic haemoglobinless teleost Chionodraco hamatus
M. Maffia*, A. Rizzello*, T. Verri*, R. Acierno*, A. Danieli*, H. Daniel† and C. Storelli*
*Department of Biological and Environmental Science and Technology, University of Lecce, Prov. le Lecce-Monteroni, 73100 Lecce, Italy and †Institute of Nutritional Sciences, Physiology and Biochemistry of Nutrition, Technical University of Munich, Hochfeldweg 2, D-85350 Freising-Weihenstephan, Germany
View other abstracts by:
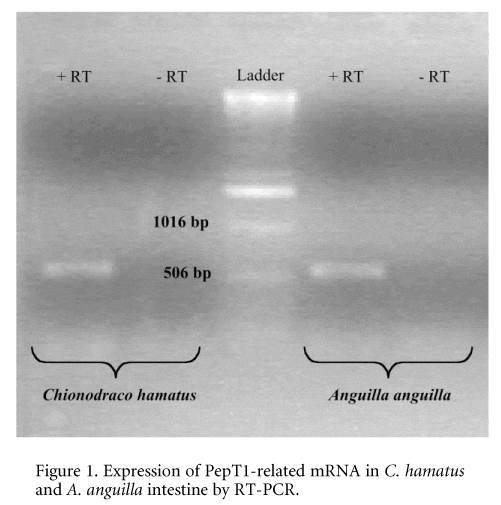
Previous studies have demonstrated that transport systems for nutrients and ions occurring at the intestine of the Antarctic teleosts (Maffia et al. 1996, 2000) exhibit specific adaptations to low temperatures. In this work we extended our research on cold-adapted transporters to a proton oligopeptide transporter located at the intestine of the Antarctic haemoglobinless teleost C. hamatus. All fish were humanely killed. The presence in the icefish intestine of a low-affinity type peptide transport belonging to the proton oligopeptide transporter (POT) superfamily (Meredith & Boyd, 2000) was assessed by reverse transcription-polymerase chain reaction (RT-PCR) using human PepT-1-specific primers (Fig. 1).
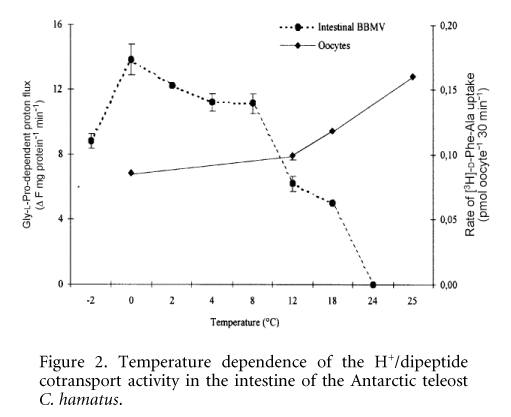
H+/dipeptide cotransport activity was measured in the intestinal brush-border membrane vesicles (BBMV) by monitoring acridine orange fluorescence changes (Verri et al. 2000). In isolated BBMV, the icefish transporter displays a substrate specificity similar to that found in eel peptide transporter and a maximal transport activity falling in a narrow range of temperatures between -2 and 5 °C. Furthermore, the functional expression of peptide transporter was performed in a heterologous system by microinjection in Xenopus laevis oocytes of poly (A)+ RNA extracted from the intestinal mucosa of C. hamatus. In oocytes injected with poly(A)+ RNA, D-Phe-Ala uptake was 3-fold higher than that measured in water-injected oocytes. The expression was dose dependent, reaching maximum activity at 40 ng of poly (A)+ RNA and time dependent, reaching maximum activity on day 3 after injection. Poly (A)+ RNA-induced transport was inhibited by 10 mM Gly-Gln. Uptake performed at different temperatures (0Ð25 °C) was maximal at 25 °C. These data suggest the presence of a low-affinity type H+/peptide transporter on the brush-border membrane of C. hamatus intestine. When the intestinal peptide transporter of the Antarctic fish is expressed in the heterologous lipid microenvironment represented by X. laevis oocyte plasma membrane, its characteristic temperature dependence -2/5 °C) is modified (Fig. 2), thus suggesting a major role of the lipid component in the transport process. However, it cannot be excluded that cold adaptation might involve structural modifications of the transporter.
All procedures accord with current National guidelines.
\"Figure 1. Expression of PepT1-related mRNA in C. hamatus and A. anguilla intestine by RT-PCR. H+/dipeptide cotransport activity was measured in the intestinal brush-border membrane vesicles (BBMV) by monitoring acridine orange fluorescence changes (Verri et al. 2000). In isolated BBMV, the icefish transporter displays a substrate specificity similar to that found in eel peptide transporter and a maximal transport activity falling in a narrow range of temperatures between -2 and 5 °C. Furthermore, the functional expression of peptide transporter was performed in a heterologous system by microinjection in Xenopus laevis oocytes of poly (A)+ RNA extracted from the intestinal mucosa of C. hamatus. In oocytes injected with poly(A)+ RNA, D-Phe-Ala uptake was 3-fold higher than that measured in water-injected oocytes. The expression was dose dependent, reaching maximum activity at 40 ng of poly (A)+ RNA and time dependent, reaching maximum activity on day 3 after injection. Poly (A)+ RNA-induced transport was inhibited by 10 mM Gly-Gln. Uptake performed at different temperatures (0Ð25 °C) was maximal at 25 °C. These data suggest the presence of a low-affinity type H+/peptide transporter on the brush-border membrane of C. hamatus intestine. When the intestinal peptide transporter of the Antarctic fish is expressed in the heterologous lipid microenvironment represented by X. laevis oocyte plasma membrane, its characteristic temperature dependence -2/5 °C) is modified (Fig. 2), thus suggesting a major role of the lipid component in the transport process. However, it cannot be excluded that cold adaptation might involve structural modifications of the transporter.\"
Figure 2. Temperature dependence of the H+/dipeptide cotransport activity in the intestine of the Antarctic teleost C. hamatus.
Where applicable, experiments conform with Society ethical requirements.