Acute mountain sickness (AMS) is a cerebral syndrome that presents at high-altitude and though preliminary neuroimaging data suggest a potential etiological role for extracellular vasogenic oedema (Hackett et al. 1998), the precise mechanisms responsible for disruption of blood-brain barrier function in hypoxia remain unresolved.
The present study therefore combined magnetic resonance imaging (MRI) with electron paramagnetic resonance (EPR) spectroscopy to directly examine, for the first time, the pathophysiological significance of free radical-mediated molecular damage to barrier function and associated neurological sequelae. Ethical approval was obtained from the Department of Medicine, University of Heidelberg. Fasting venous and cerebrospinal fluid (CSF) samples were obtained in the lateral decubitus position from twenty two healthy subjects aged 24 ± 2 (mean ± S.D.) years old after 16-18h passive exposure to normobaric hypoxia (12% O2). Serum was analysed for the ascorbate free radical (A-{special}–) or mixed ex-vivo with the diamagnetic spin trap, α-phenyl-tert-butylnitrone (PBN) prior to room temperature EPR spectroscopy (Bruker, EMX). Brain-specific proteins (neuron-specific enolase, S-100β), proinflammatory cytokines (TNF-α, IL-1β, IL-6) and vascular endothelial growth factor were also assayed via routine techniques. Barrier function was examined using an established clinical technique (CSF/serum concentration quotient for albumin, IgG, IgA, IgM). Dual echo-, diffusion-weighted imaging [T2-relaxation time/apparent diffusion coefficient (ADC)] and T1-3D (volumetry) MRI sequences were determined in specific regions of interest in normoxia and after ~16 h of hypoxia. Clinical AMS was confirmed at 18 h if subjects presented with a Lake Louise self-assessment and clinical score of ▓ge│5 points combined with an Environmental Symptoms Questionnaire Cerebral Score ▓ge│0.7 points. Data were mathematically examined for distribution normality and selective differences investigated using an independent samples t or Mann Whitney U test where appropriate.
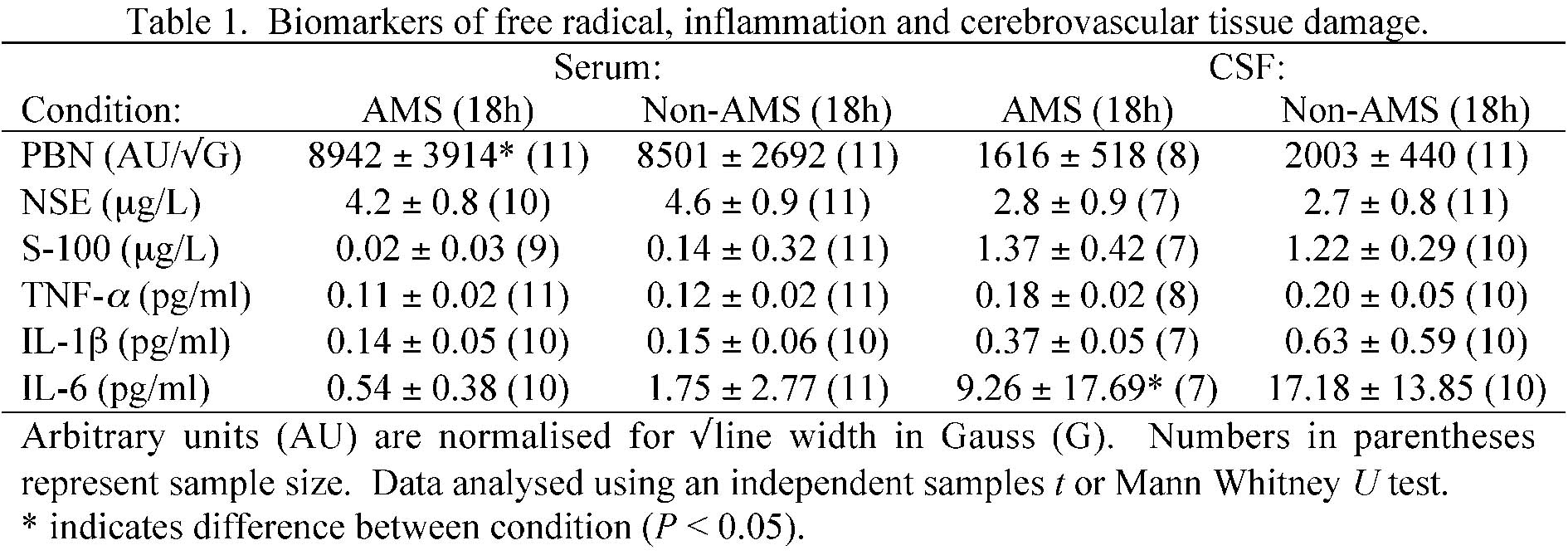
PBN spin-adducts recovered in serum and CSF were later identified via in-vitro experimentation and computer simulation experiments as lipid-derived alkoxyl/alkyl radicals [aN = 13.6-14.2 Gauss (G) and aãHãβ = 1.5-2.3 G] formed ÊdownstreamË subsequent to the oxidative catalysis of iron [Fe(III)-EDTA]. Eleven subjects diagnosed with AMS (50%) presented with a selective elevation in serum A-{special}– (AMS: 1638 ±274 vs. non-AMS: 1204 ±271 arbitrary units/Ã{special}G, P < 0.05) and decrease in the CSF concentration of IL-6 (AMS: 9.26 ± 17.69 pg/ml vs. non-AMS: 17.19 ± 13.85 pg/ml, P < 0.05). Differences in absolute brain tissue volume (AMS: +0.7 ± 0.4 vs. non-AMS: +0.5 ± 0.4%) despite T2-prolongation in the splenium of the corpus callosum, ADC, lumbar pressure (AMS: 11.8 ± 3.7 vs. non-AMS: 12.2 ± 5.1 cm/H2O) and barrier function were unremarkable (P > 0.05).
In conclusion, these findings provide the first direct evidence for spin-trapped oxygen/carbon-centered free radicals in human CSF. Selective changes in brain morphology subsequent to free radical-mediated molecular damage to barrier function do not appear to be an initiating factor in AMS.