Coherent membrane potential oscillations in neuronal networks provide a common ‘clock’ for individual neurons which can discharge at defined time points within the cycle. In the mammalian hippocampus, this ‘phase coding’ seems to be of particular importance during combined theta/gamma oscillations (5-10 and 30-100 Hz, respectively) and during very fast (~200 Hz) oscillations called ‘ripples’. Fixed temporal sequences of consecutively firing place-encoding neurons are established during theta/gamma activity when a rat explores a new environment and are replayed at a faster time scale during ripples at rest or sleep. These observations have given rise to the idea that ripples are involved in memory consolidation (reviewed in Draguhn et al. 2000).
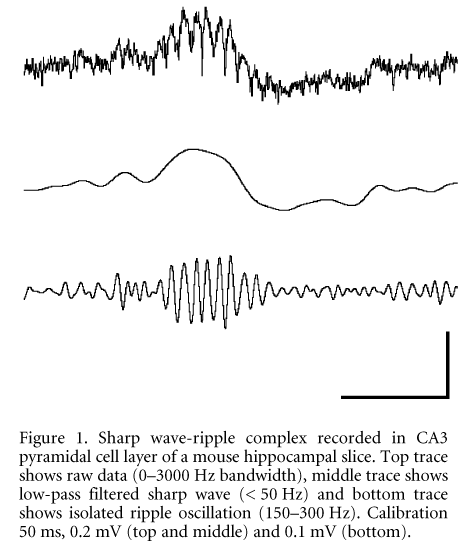
While the cognitive function of ripples can be best studied in the living animal, the underlying mechanisms of synchronisation are most easily analysed in in vitro preparations. We have therefore studied high-frequency (~200 Hz) oscillations in rat and mouse hippocampal slices. Hippocampi were dissected and sliced after brain removal in deep ether anaesthesia (as permitted by the Berlin local government). Extracellularly recorded field potentials present brief periods of spontaneously occurring fast potential oscillations in the principal cell layers. The waveforms, however, are strikingly different between rat and mouse tissue: in slices from juvenile or adult rats, we regularly observed series of 3-10 negative-going deflections reminiscent of small (0.02-0.4 mV) population spikes (Draguhn et al. 1998). In slices prepared from mice, the fast negative deflections are superimposed on positive-going waves of 20-80 ms duration (Fig. 1). The global pattern and frequency content of these potential fluctuations is very similar to sharp wave-ripple complexes in vivo (Buzsçki et al. 1992) and therefore we term the spontaneously occurring network activity in mouse hippocampal slices ‘sharp wave-ripple complexes in vitro‘ (SW-R).
Recordings with multiple electrodes revealed that SW-R propagate from CA3 towards CA1 and to the subiculum. Besides this propagation along the hippocampal output loop, we also found a ‘backward’ propagation towards the hilus and the dentate gyrus. Propagation velocity was around 2 cm s-1, far below the axonal conductance velocity in the hippocampus. The laminar profile revealed most prominent signals in the pyramidal cell layer and a phase reversal of sharp waves and ripples between stratum pyramidale and stratum radiatum. These spatial properties are very similar to the behaviour of sharp wave-ripple complexes in vivo.
SW-R do depend on fast chemical synaptic transmission: CNQX completely blocked SW-R activity while the NMDA-receptor antagonist DL-APV had no visible effect. Likewise, SW-R were completely blocked when we suppressed GABAergic inhibition with bicuculline or gabazine. In these disinhibited slices we regularly observed large interictal-like discharges with superimposed oscillations at ~200 Hz.
We had previously suggested that ripples are synchronised by electrical coupling via gap junctions. We therefore applied three different uncoupling agents (carbenoxolone, octanol and quinine) and found that all of them strongly reduced or even abolished the SW-R activity. Interestingly, ripples were more strongly suppressed than the underlying sharp waves, indicating that 200 Hz oscillations depend more critically on gap junctions than the slow excitatory waves.
We finally recorded the intracellular potential of CA1 pyramidal neurons (n = 13) together with the nearby field potential. Sharp waves were accompanied by depolarising, hyperpolarising or biphasic potential fluctuations in all pyramidal cells. However, even during depolarising responses membrane potential never reached threshold for action potential generation. Voltage dependence of the signals revealed a negative (< -60 mV) reversal potential, consistent with a dominant GABAA receptor-mediated chloride conductance. When we depolarised the cells sufficiently to generate regular firing, action potentials usually ceased during SW-R, again indicating that the net effect of SW-R on most pyramidal cells is inhibitory.
In summary, sharp wave-ripple complexes can be well studied in mouse hippocampal slices and show a spatial distribution, propagation and frequency content which is very similar to sharp wave-ripple complexes in vivo. The signals are generated by chemical as well as by electrical coupling of neurons and result in a strong inhibition of most local pyramidal cells.
This work was supported by the DFG (Dr 326/1-3).