Since their description in skeletal muscle (Guharay & Sachs, 1984) stretch-activated channels (SACS) also appear in heart (Craelius et al. 1988). Many of their electrophysiological characteristics have an equivalence in intact heart, where monophasic action potentials (MAPs) are used for qualitative insights into electrophysiology. The preparations include isolated perfused heart – frog (Lab, 1978), mammalian ventricle (Franz et al. 1985; Hansen et al. 1990; Dick & Lab, 1998). Notably, there is a type of reversal potential (Lab, 1978; Zabel et al. 1996) where a stretch early in the action potential produces a repolarising tendency, whereas if late it produces depolarisation. At the ‘reversal potential’ the voltage time course remains unchanged. The equivalence extends not only to intact heart in situ, in mammalian experimental preparations (Dean & Lab, 1987), but it also applies in man (Taggart et al. 1988). Importantly, the mechanical perturbation can give rise to clinically relevant premature beats. The likelihood that SACs are operative is further strengthened: gadolinium, a proposed SAC blocker, reduces the effects of stretch (Hansen et al. 1990). The same applies to streptomycin (Gannier et al. 1994; Eckardt et al. 2000). We have found, however, that streptomycin’s effects can be heterogeneous in intact atrium (Babuty & Lab, 2001).
In the face of the foregoing persuasive studies, how can one contemplate the possibility that SACS are overrated in producing the electrophysiological changes in intact heart? This can be facilitated – correlation does not mean causality – a source of many a pitfall in interpretation. What are the alternatives?
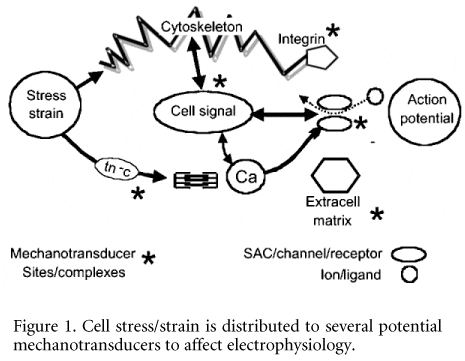
Essentially one has to reflect on alternative mechanoelectric transducers in heart. Any membrane channel associated with the cytoskeleton could be a candidate (see Fig. 1, SACs/channels/ receptors), for stress-strain could be transmitted to the channel in this way. There is evidence that the ATP-activated potassium (KATP) channel is not only attached to the cytoskeleton, but it has been suggested to be mechanosensitive (Van Wagoner, 1993): similarly the L-type Ca channel (Matsuda et al. 1996). We have shown (Gu et al. 2002) that several ion channels are fixed (probably via cytoskeleton) in Z grooves and around T-tubule openings. This means that they could be subject to transmitted forces. Although this makes them candidates for mechanoelectric transduction, we have yet to investigate this aspect. However, several other channels may be mechanosensitive, including sodium channels (Tabarean et al. 1999) and potassium channels such as TREK-1 (Maingret et al. 2002) (which appears not to involve the cytoskeleton), and IKAA (Kim, 1992), ion exchangers (Perez et al. 2001). However, invoking all the foregoing to substitute the SAC mechanism for explaining the observations in intact heart violates ‘Occam’s razor’ which needs the fewest hypotheses to explain an observation. If force transmission via the cytoskeleton has multi-channel effects, it should be operating on a beat-to-beat basis, continuously modulating the action potential. If so, perturbing the cytoskeleton should have electrophysiological effects. Pilot experiments in our laboratory show that depolymerising cytoskeletal f-actin modulates the MAP.
Another mechanoelectric transducer in the actively beating heart is via the binding constant of troponin c (tn-c) for calcium (see Fig. 1). Papillary muscle shortening reduces the binding constant for Ca and this increases intracellular Ca to influence transmembrane charge movement (Lab et al. 1984). Isometric contraction reduces the action potential duration (APD), whereas shortening prolongs it – sometimes taking the form of an afterdepolarisation which can, as in stretch of intact heart described above, produce premature beats (Kaufmann et al. 1971). Superficially, it appears difficult to supplant the SAC mechanism with this one, for intraventricular distension should produce a sustained stretch and isovolumic (isometric) contraction. However, contraction at the segment level is counter-intuitive. The segment often stretches early in systole (accompanied by MAP reduction) and shortens much later (accompanied by MAP prolongation, or afterdepolarisation). That is, the tn-c mechanism mimics the SAC mechanism.
Alternative mechanotransducers also reside in cell ‘signalsomes’ (see Fig. 1, cell signal, SACs/channels/receptors). One notable example is the β receptor. We have shown that β agonism or manipulation can significantly modulate mechanically induced electrophysiological changes (Horner et al. 1996). Raised intraventricular pressure results in catecholamine release from the ventricle (La Farge et al. 1970) and conceivably ventricular mechanical changes affect the cell signal chain involving β agonist/receptor, ATP, cAMP and Ca channels
Finally, other cell ‘signalsomes’ have also been implicated as being mechanosensitive and, mostly via intracellular Ca, affect membrane electrophysiology. These include AT2 and ET1 receptors (see brief overview: Lab, 1999) which would affect PLC via Gq. This can alter intracellular Ca, firstly, via IP3 and SR Ca release, and secondly through PLC to facilitate membrane translocation (DAG/RACK/PKC) to influence Na-H exchange, and so intracellular Na via Na-Ca exchange. An under-investigated but potentially important mechanism invokes mechanically induced changes in ATP (Kawakubo et al. 1999)
In conclusion, it is conceivable that the plethora of channels, exchangers, and cell ‘signalsomes’ that exist in heart to transduce mechanical to electrical energy render SACs to just being one of many contributors to cardiac mechanoelectric feedback or transduction, and not the main one.