University of Leeds (2002) J Physiol 544P,
S305
Research Symposium: Mathematical modelling of mechanical effects on action potential duration in heterogeneous myocardium
Olga Solovyova, Nathalie Vikulova, Vladimir Markhasin, Penelope J. Noble†, Alan Garny† and Denis Noble†
*Ekaterinburg Branch of the Institute of Ecology and Genetics of Microorganisms, Pervomayskaya 91, Ekaterinburg, Russia and †University Laboratory of Physiology, Parks Road, Oxford OX1 3PT, UK
View other abstracts by:
Myocardial heterogeneity affects cardiac mechano-electrical function, but how exactly remains unclear. This is partially due to the lack of simplified models for the study of this subject. We developed a fundamental model of cardiac heterogeneity (duplex), consisting of two separate muscle segments that are mechanically coupled (in series or parallel). Muscle segments may either be (1) biological samples (trabeculae), (2) mathematical models, or (3) a combination thereof (Solovyova et al. 2002).
Here, we studied mechano-electrical interactions in heterogeneous ‘in series’ duplexes, consisting of two virtual muscles (VMs). Each VM constitutes a mathematical model of guinea-pig ventricular electromechanical activity (Garny et al. 2001), developed by the Ekaterinburg and Oxford teams. Mechanical parameters for individual VMs were set to simulate a ‘fast’ and a ‘slow’ VM (Fig. 1A). In isolation, action potential duration (APD) was found to be longer for the slow muscle than for the fast one (in spite of identical parameters for the electrical blocks of both muscles). This difference in APD was produced by the difference in cross-bridge kinetics influencing Ca2+ dissociation from Troponin C, which affected Ca2+ transient and, consequently, Na+-Ca2+ exchange current.
We conclude that (i) myocardial electromechanical hetero-geneity is an important determinant of ED; (ii) that this may be studied in reduced models of heterogeneity, consisting of only two elements; and (iii) that our virtual duplex model may provide a framework for developing experimentally testable hypotheses on this subject.
This work is supported by The Wellcome Trust and the Russian Foundation for Basic Research.
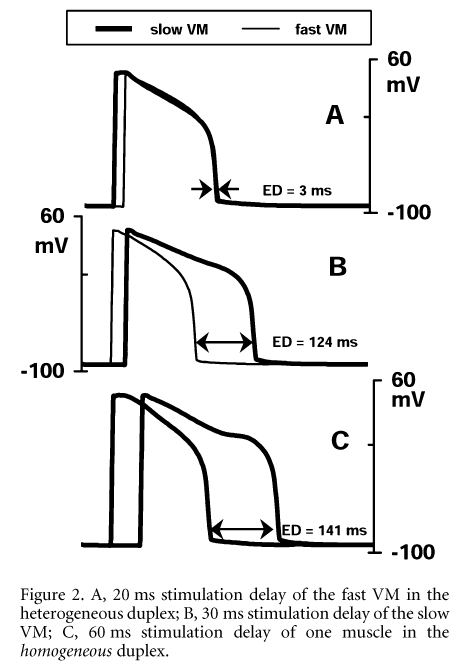
Figure 1. Electromechanical characteristics of individual VMs. A, in isolated conditions; B, within 'in series' duplex. From top to bottom: isometric force (arbitrary units); muscle length normalized by Lmax; [Ca-TnC]; [Ca2+]i; AP.In the duplex setting, we investigated isometric contractions of in series heterogeneous duplexes. External length of the duplex was fixed, whereas length of individual elements varied dynamically, due to the asynchronous mechanical activity of components. This internal mechanical interaction produced alterations in both mechanical and electrical activity of each muscle (see Fig. 1B for comparison with control behaviour in isolated conditions shown in Fig. 1A). Namely, APD of the fast muscle decreased further, while APD of the slow one increased, causing an increase in electrical dispersion (ED), defined as the time difference between 90 % repolarization. This ED may be modulated by introduction of a time lag (ΔTstim) in electrical activation of duplex elements, to simulate the differences in the speed of electrical (up to 3.5 m s-1) and mechanical (up to 350 m s-1) 'conduction' in cardiac tissue. Not unexpectedly, ED decreased when stimulation of the fast muscle was delayed and vice versa. However, there was a significant difference between the ΔTstim value and the effect on ED (Fig. 2A and B). Furthermore, applying ΔTstim for individual muscles in homogeneous duplexes (pairs of either fast or slow muscles) caused ED that significantly exceeded the delay value (Fig. 2C). Model analysis showed that these alterations in APD of mechanically interacting muscles were caused by non-linear mechanodependent changes in their Ca2+ kinetics, affecting ED mainly via the Na+-Ca2+ exchange current during the late plateau phase of the AP.\"
Figure 2. A, 20 ms stimulation delay of the fast VM in the heterogeneous duplex; B, 30 ms stimulation delay of the slow VM; C, 60 ms stimulation delay of one muscle in the homogeneous duplex.\"
Where applicable, experiments conform with Society ethical requirements.

![Figure 1. Electromechanical characteristics of individual VMs. A, in isolated conditions; B, within 'in series' duplex. From top to bottom: isometric force (arbitrary units); muscle length normalized by Lmax; [Ca-TnC]; [Ca2+]i; AP.In the duplex setting, we investigated isometric contractions of in series heterogeneous duplexes. External length of the duplex was fixed, whereas length of individual elements varied dynamically, due to the asynchronous mechanical activity of components. This internal mechanical interaction produced alterations in both mechanical and electrical activity of each muscle (see Fig. 1B for comparison with control behaviour in isolated conditions shown in Fig. 1A). Namely, APD of the fast muscle decreased further, while APD of the slow one increased, causing an increase in electrical dispersion (ED), defined as the time difference between 90 % repolarization. This ED may be modulated by introduction of a time lag (ΔTstim) in electrical activation of duplex elements, to simulate the differences in the speed of electrical (up to 3.5 m s-1) and mechanical (up to 350 m s-1) 'conduction' in cardiac tissue. Not unexpectedly, ED decreased when stimulation of the fast muscle was delayed and vice versa. However, there was a significant difference between the ΔTstim value and the effect on ED (Fig. 2A and B). Furthermore, applying ΔTstim for individual muscles in homogeneous duplexes (pairs of either fast or slow muscles) caused ED that significantly exceeded the delay value (Fig. 2C). Model analysis showed that these alterations in APD of mechanically interacting muscles were caused by non-linear mechanodependent changes in their Ca2+ kinetics, affecting ED mainly via the Na+-Ca2+ exchange current during the late plateau phase of the AP.\"](https://static.physoc.org/app/uploads/2019/01/22203830/S305-f1.gif)