The principal physiological function of the prostate gland is the synthesis, accumulation and secretion of citrate. In prostate cancer, citrate production levels are significantly reduced as a result of the altered cellular metabolism and bioenergetics (Costello & Franklin, 1997, 2000). Na+,K+-ATPase function is essential for citrate production since the inward Na+ gradients generated by Na+,K+-ATPase are utilized for the Na+-dependent uptake of aspartate, a major substrate for citrate synthesis. Accordingly, the objective of this study was to compare the localization and relative abundance of Na+,K+-ATPase isoforms in normal dog prostate, benign prostatic hyperplasia (BPH) and prostatic adenocarcinoma (PCa) in order to see if reduced citrate levels in PCa are accompanied by changes in Na+,K+-ATPase expression.
Dog prostate glands were obtained from animals humanely killed for clinical reasons. Prostates were fixed in formaldehyde and embedded in paraffin before sectioning and immunohistochemical staining using a panel of well-characterized monoclonal and polyclonal antibodies raised against known Na+,K+-ATPase subunit isoforms.
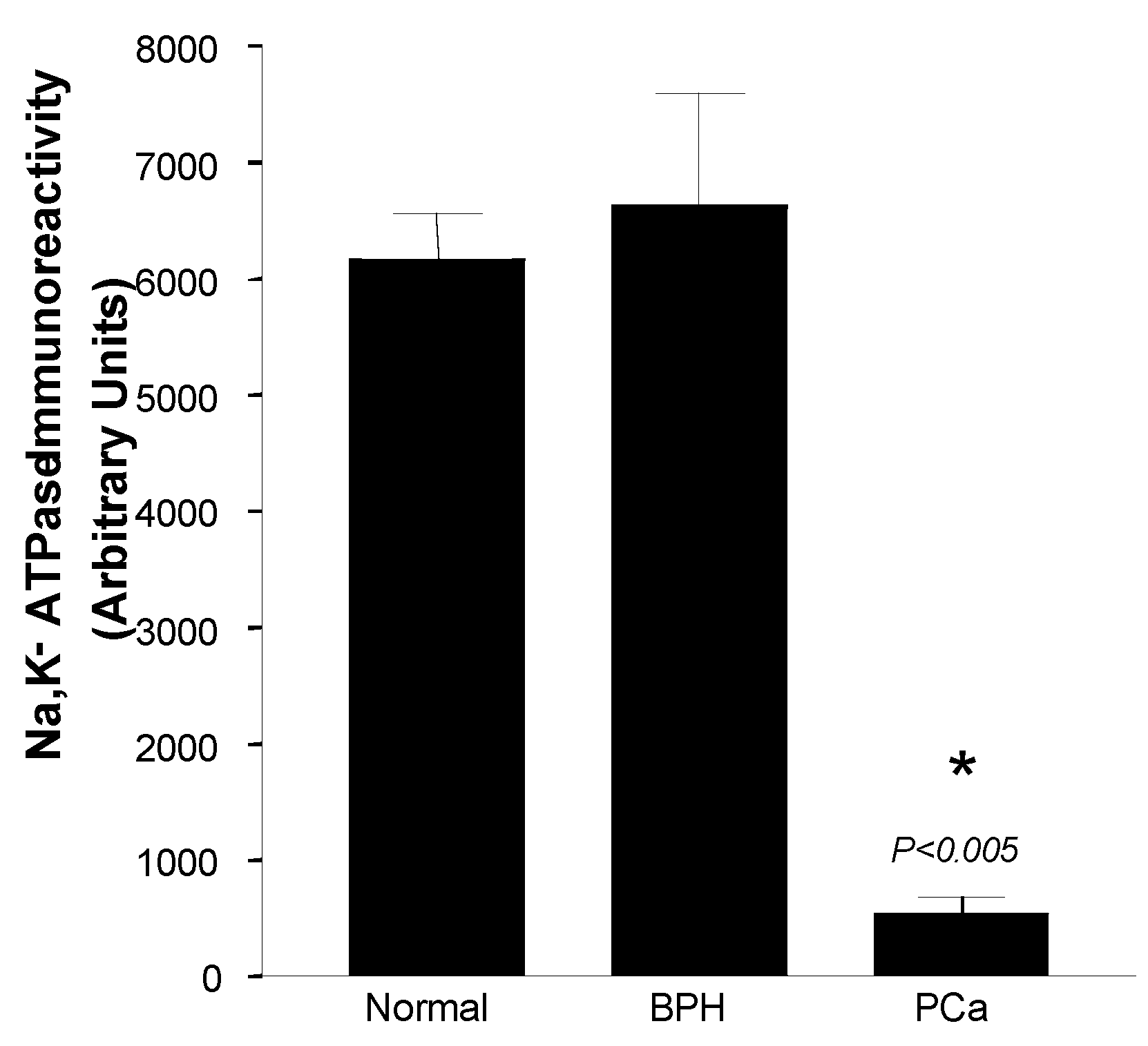
Expression of Na+,K+-ATPase α1, β1 and β2 and, was observed in the lateral and basolateral plasma membrane domains of prostatic epithelial cells in normal and BPH prostates. We did not detect the λ subunit of Na+,K+-ATPase in any of the normal, BPH or PCa tissues examined. The α1 isoform was detected in abundance but there was no evidence of α2 or α3 isoform expression in prostatic epithelial cells. In advanced PCa prostates Na+,K+-ATPase isoform expression was reduced by more than 90 % compared to normal and BPH glands (Fig. 1). Dog prostate Na+,K+-ATPase consisted primarily of α1/β1 and α1/β2 isoenzymes. These isoform proteins were predominantly localized to the basolateral plasma membrane domains of prostatic epithelial cells in normal and BPH specimens. The abundant basolateral immunostaining observed in normal and BPH glands was significantly attenuated in PCa sections as revealed by image analysis. The metabolic transformation of zinc-accumulating citrate-producing normal prostate epithelial cells to citrate-oxidizing malignant cells has important implications for cellular bioenergetics, and metabolism and appears to be accompanied by a down-regulation of Na+,K+-ATPase.
This study was supported by a grant from the Pet Plan Charitable Trust (UK).