Immunocytochemical and functional studies suggest that L-type Ca2+ channels are located predominantly in the t-tubules of cardiac ventricular myocytes. The purpose of the present study was to compare modulation of L-type Ca2+ current (ICaL) by Ca2+ in the t-tubules (i.e. in control cells) and surface sarcolemma (i.e. in detubulated (DT) cells.
Wistar rats were killed humanely using a Schedule 1 method. Ventricular myocytes were enzymatically isolated, and detubulated using formamide as described by Kawai et al. (1999). The whole cell configuration of the patch clamp technique was used to record ICaL. TEA/Cs-containing solutions were used, to avoid contamination by other currents; membrane potential was held at -80 mV and test pulses to 0 mV applied. Experiments were performed at 22-24 °C.
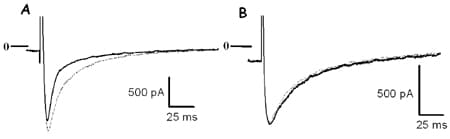
The rapid component of inactivation of ICaL was significantly faster in control cells (t{special} = 9.5 ± 0.6 ms, mean ± S.E.M., n = 10) than in detubulated cells (16.8 ± 2.8 ms, n = 7, P < 0.05, Student’s unpaired t test), and frequency-dependent facilitation was present in control cells (10/12) but not in detubulated cells (12/12, see Fig. 1). Inactivation and facilitation of ICaL are known to depend on local Ca2+ entry and release. Ryanodine (30 µM) slowed the fast inactivation phase of ICaL in control cells so that it was no longer statistically different from in DT cells (control t{special}: 22.7 ± 2.1 ms, n = 10; detubulated: 23.8 ± 2.0 ms, n = 7; NS, unpaired t test) and abolished frequency-dependent facilitation in control cells. Formamide treatment of atrial myocytes, which lack t-tubules, did not change the fast inactivation phase of ICaL (control t{special}: 9.8 ± 1.4 ms, n = 7; formamide-treated: 10.0 ± 1.6 ms, n = 7; NS, unpaired t test).
It appears likely that Ca2+-dependent modulation of ICaL is due to local Ca2+ entry and release rather than bulk cytoplasmic Ca2+ (see for review Dirksen, 2002). Thus the observation that modulation of ICaL by Ca2+ released from the SR is most marked at the t-tubules (i.e. in control cells) suggests that this difference may be due to differences in the structure of the dyad at the t-tubule and surface membrane.
This work was supported by project grants from the Wellcome Trust and British Heart Foundation, and a short-term travel grant to L.S. from the Wellcome Trust.