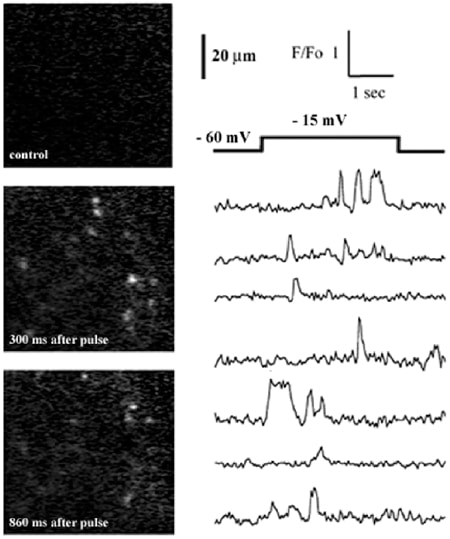
Patch-clamp recording (Neher & Sakmann, 1976) has revolutionized our ability to study the function of ion channels, but suffers some limitations: notably, it offers little spatial information and cannot provide independent readout from multiple channels. Optical techniques hold promise as a complementary approach, and fluorescence imaging has resolved calcium flux through individual channels (Zou et al. 1999; Wang et al. 2001; Demuro & Parker, 2002). Further improvements should be possible by localizing calcium signals more tightly around the channel mouth, where changes in calcium concentration are greatest and most rapid. For this purpose total internal reflection fluorescence microscopy (TIRFM) (Axelrod, 2003) is ideal, because the evanescent wave formed by TIR at a glass-water interface extends only a few hundred nanometres. To explore this idea, we imaged calcium flux through N-type channels expressed in Xenopus oocytes.
Oocytes were injected with cRNAs for the α1B-d and β3 subunits of N-type calcium channels (a kind gift of Dr Dianne Lipscombe). After 4-6 days oocytes were injected with fluo-4 dextran (40 µM final concentration), the vitelline envelope was manually stripped, and oocytes were placed in a chamber constructed from a fresh coverglass and O ring and bathed in Ringer solution including 6 mM calcium. We imaged through an Olympus X 60 NA 1.45 TIRFM objective in an inverted microscope equipped with an argon ion laser and intensified CCD camera. Depolarizing pulses (applied via a two-electrode voltage clamp) evoked random, pulsatile fluorescence signals (Fig. 1) from many local regions of the cytosol. These are likely to reflect calcium flux through individual channels, because they displayed stochastic kinetics, were absent in non-mRNA-injected oocytes, increased in frequency with a voltage dependence mirroring the gating of N-type channels, and were blocked by 2 µM nickel. Simultaneous measurements could be obtained from >100 channels, with a spatial resolution of < 1 µm and a kinetic resolution (30 ms) at present limited by our video-rate camera.
We anticipate that TIRFM imaging will find wide applications in neuroscience research and high-throughput screening for measurement of functional kinetics and spatial localization of calcium-permeable channels.
This work was supported by NIH grant GM48071