It has long been postulated that there are controls on renal phosphate handling in addition to the well-understood vitamin D/parathyroid hormone (PTH) axis. In oncogenic osteomalacia, patients have a tumour-related increase in urinary phosphate loss that resolves when the tumour is resected. Matrix extracellular phosphoglycoprotein (MEPE), a protein isolated from a patient with oncogenic osteomalacia (Rowe et al. 2000), is a candidate regulator of phosphate homeostasis. It is known to be synthesized by osteoblasts (Argiro et al. 2001) but its renal actions in intact animals are unclear.
Male Sprague-Dawley rats were anaesthetised with intraperitoneal sodium thiopentone (100 mg kg-1), prepared for clearance studies and infused intravenously with 0.9 % NaCl solution at 4 ml h-1. After a 1 h control period the infusion was changed to include PTH (40 µg kg-1 h-1) or MEPE (600 µg kg-1 h-1), or continued as saline alone, for the following 2 h. Urine phosphate loss was measured hourly. Glomerular filtration rate was determined by clearance of tritiated inulin and blood pressure was measured continuously. At the end of the experiment the animal was humanely killed.
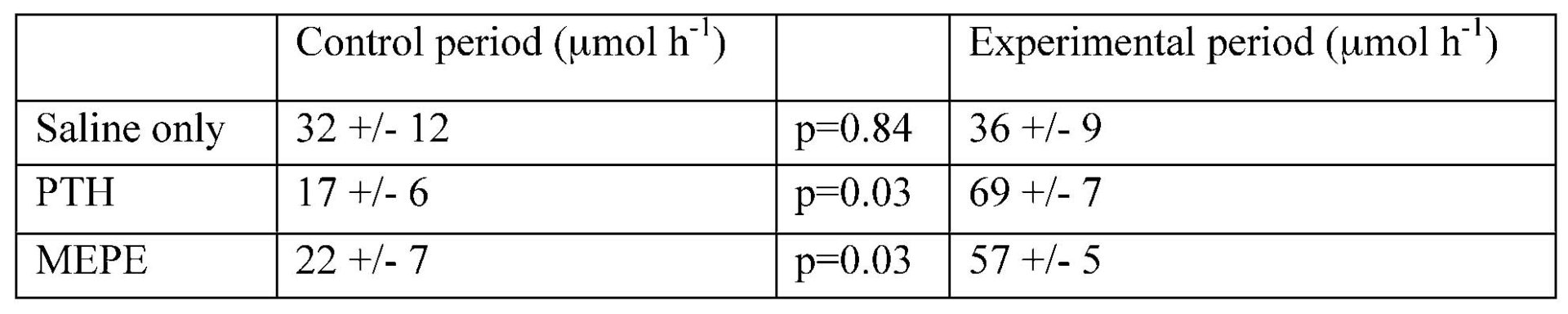
Differences in phosphate excretion between groups during the control period were not statistically significant (one-way ANOVA). Phosphate excretion was significantly increased in animals infused with either PTH or MEPE, but not in those infused with saline alone. There was no significant change in GFR in any group, and although in each group there was a slight decline in blood pressure over time, no differences were seen between groups.
These results show that infusion of MEPE into intact rats causes phosphaturia. The mechanism of this effect and whether it may be of physiological as well as pathological significance remain to be determined.
This work was supported by the National Kidney Research Fund. We thank Russ Blacher at Acologix for the supply of MEPE.