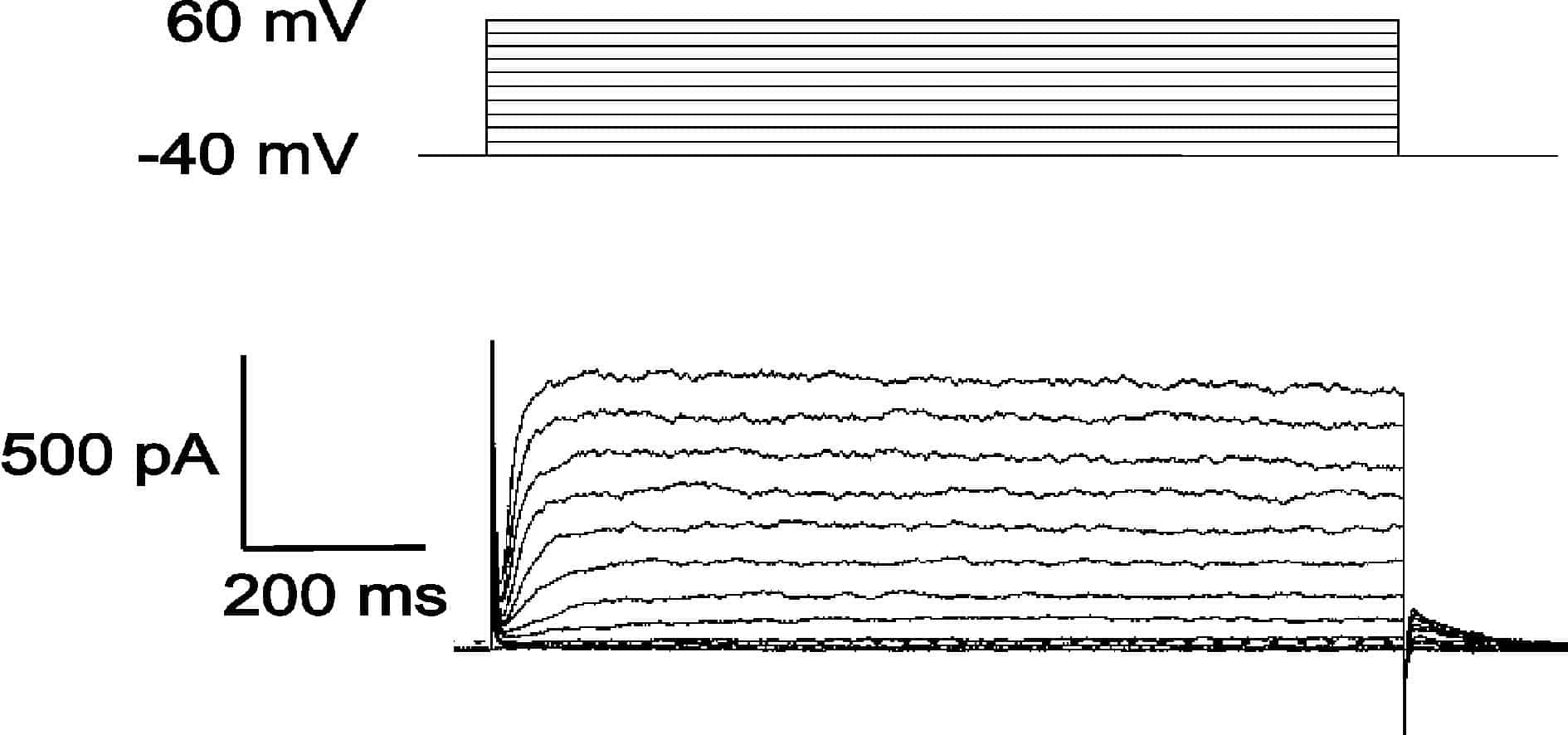
The liver fluke, Fasciola hepatica, relies on highly co-ordinated motor activity to enter and remain within the bile ducts of its host. Understanding the control of this mechanical activity may suggest new therapeutic strategies with which to combat these and similar parasites. Since mechanical activity in many muscle types is triggered by excitation of the cell membrane, we set out to characterise some of the relevant ionic currents.
Rats infected with F. hepatica were humanely killed and flukes were extracted from the biliary tree. Cells were isolated from the ventral suckers using successive incubations at 37 °C in Ca2+-free Hedon-Fleig solution containing dithiothreitol (5 mM) and bovine serum albumin (0.1 %) to which papain (2 mg ml-1) was added during the first incubation and trypsin (2 mg ml-1) during the second. Muscle fibres were then released by gentle trituration in enzyme-free solution and superfused with Ca2+-containing modified Hedon-Fleig solution at room temperature. They were voltage clamped using the perforated patch technique with amphotericin B (600 µg ml-1) dissolved in pipette solution containing KCl (53 mM) and potassium gluconate (80 mM). The statistical significance of differences in summarised data (means ± S.E.M.) was assessed using Student’s paired t test.
Depolarisation from a holding potential of -40 mV activated outward currents (Fig. 1). The kinetics of activation were voltage dependent, with the time required for the current to reach 80 % of its peak value falling from 57 ± 11 ms at 0 mV to 16 ± 2 ms at +60 mV (P < 0.005, n = 7). Tail currents recorded on repolarisation to different potentials after a 200 ms activation step to +60 mV reversed direction at -69 ± 3 mV under control conditions (EK = -89 mV). This reversal potential shifted to -23 ± 1 mV when EK was raised to -37 mV (P < 0.00005, n = 6). The deactivation kinetics of these tail currents were also strongly voltage dependent, the time required for 80 % decay falling from 123 ± 9 ms at -20 mV to 6 ± 1 ms at -110 mV (P < 0.0001, n = 6). The peak outward current was reduced by 77 ± 6 % in the presence of tetrapentylammonium chloride (1 mM; P < 0.0005, n = 5), by 59 ± 4 % in the presence of nimodipine (30 µM; P < 0.005, n = 7) and by 52 ± 9 % in the presence of verapamil (30 µm; P < 0.001, n = 8). Superfusion with BAPTA-AM (50 µM) also inhibited the peak outward current by 44±16% (P < 0.05, n = 5).
These results suggest that voltage- and Ca2+-sensitive K+ currents are likely to play a role in shaping membrane potential changes in these cells.
D.K. was supported by an International Research Fellowship from the Wellcome Trust.