Secretion of fluid into the brain requires transfer of solutes and water across the luminal and abluminal membranes of the endothelial cells lining the brain capillaries. The present study seeks to identify the transporters involved in the movement of Na+ and/or Cl– in these endothelial cells by measuring changes in intracellular pH (pHi).
Brain endothelial cells were grown from isolated rat brain microvessels (rats were humanely killed) and maintained in primary culture. For pHi measurement, cells were plated on glass coverslips, loaded with BCECF and their fluorescence then measured in a fluorimeter.
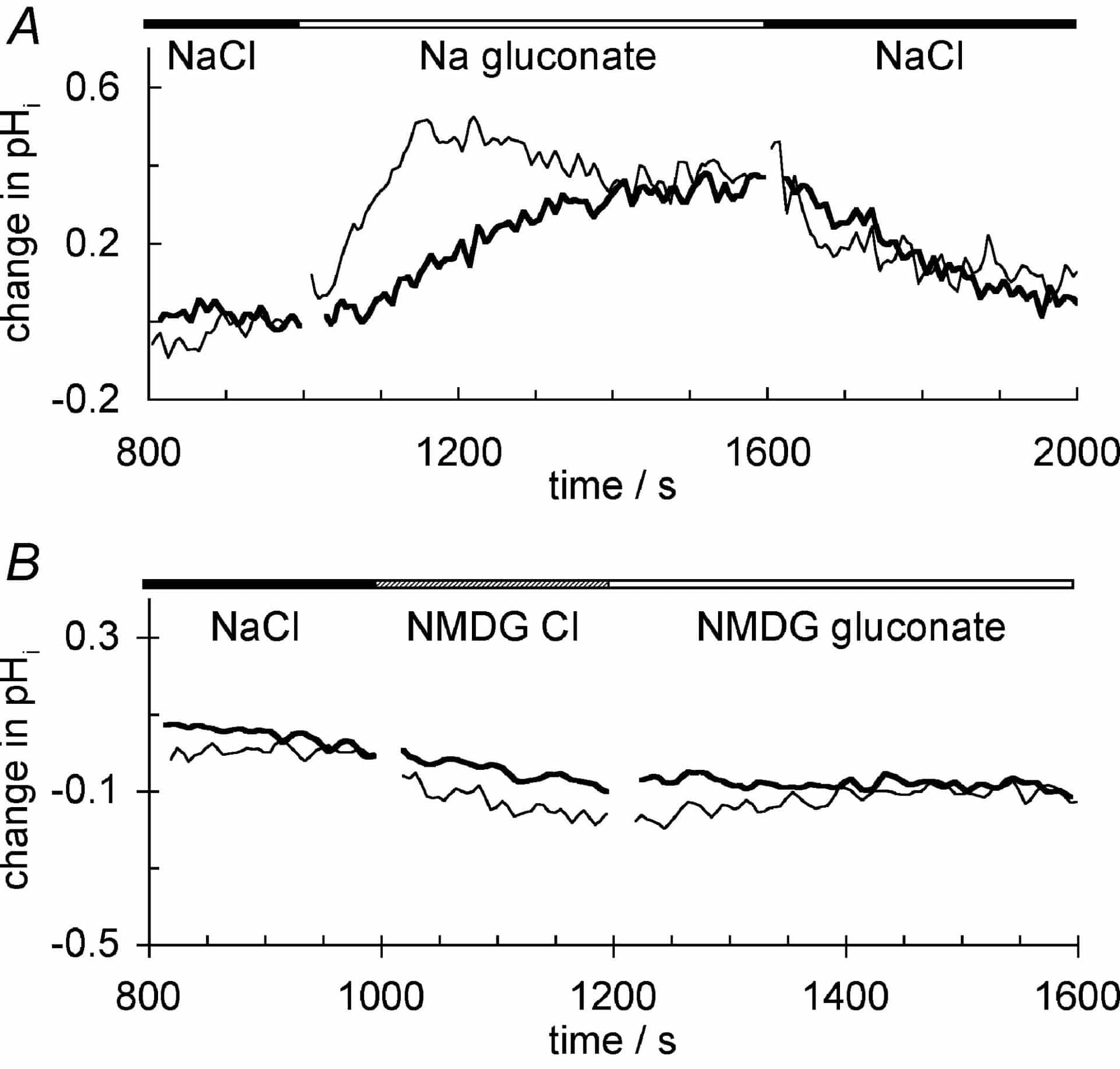
Cells in buffer containing 10 mM Hepes and 5 % CO2/HCO3–, pH 7.4 maintained a pHi of 7.32 ± 0.03 (mean ± S.E.M., n = 32) (Taylor et al. 2002). Following replacement of Na+ with NMDG+, a slow progressive acidification was observed with nearly constant rate of decrease in pHi for at least 600 s. This rate of decrease was similar in the presence or absence of CO2/HCO3– (0.032 ± 0.002 pH units min-1, n = 5 vs. 0.027 ± 0.003 pH units min-1, n = 3, P = 0.23, Student’s unpaired t test). We reported previously that replacement of Cl– with gluconate– in the external buffered solution did not alter pHi. However, further studies have shown that the effects of replacement of Cl– depend on the concentrations of Na+ and HCO3– present in the buffer solutions (see Fig. 1). In Hepes buffer containing the trace HCO3– derived from the air (estimated as ~100 µM), replacement of external Cl– by gluconate– increased pHi by 0.41 ± 0.03 pH units (mean ± S.E.M., n = 8) with t1/2 = 191 ± 9 s (thick trace in A). Such a shift is consistent with replacement of total intracellular Cl– by base equivalents which titrate the intracellular buffers. In Hepes containing 2 mM HCO3– the rate of pHi increase was faster, t1/2 = 97 ± 3 s (n = 3), but the extent of the increase was similar (thin trace in A). In buffer containing 22 mM HCO3–, the response was too fast to resolve (t1/2 < 10 s), appearing as a step increase in pHi (0.15 ± 0.01 pH units, n = 4) These are consistent with Cl–-HCO3– exchange as the mechanism of loss of Cl– and increase in pHi. When Na+ was replaced by NMDG+ before withdrawal of Cl–, the changes in pHi seen in Na+-containing Hepes- or HCO3–-buffered solutions were abolished whether the external solution was buffered with Hepes alone (thick trace in B) or with Hepes plus 5 % CO2/HCO3–.
These results indicate the presence of Na+-dependent Cl–-HCO3– exchange in endothelial cells derived from the rat blood-brain barrier.
This work was supported by the Sir Jules Thorn Charitable Trust