Effects of mechanical stimulation of cardiomyocytes on sarcoplasmic reticulum (SR) Ca2+ handling are not well understood. Ion flux through stretch-activated channels (SACs) could cause a net increase in SR Ca2+ load, either directly via Ca2+ entry, or secondary to Na+ influx (affecting Na+-Ca2+ exchange). In contrast, Gamble et al. (1992) report a reduced SR Ca2+ content in rat papillary muscle after 60 s of diastolic stretch. The sub-cellular mechanisms of this response are not clear. In this study, we use remote-controlled carbon fibres to axially stretch ventricular myocytes.
Ventricular myocytes were isolated from guinea-pigs (350-400 g) humanely killed by cervical dislocation. Cells were Fura-2 loaded (1.25 µM, 5 min), conditioned by field-stimulation (2 Hz), and Ca2+ transients and sarcomere length were simultaneously recorded (IonOptix) at 37 °C. After obtaining a steady state, pacing was interrupted for between 5 and 60 s either at resting length or during stretch by 5-10 %. Post-rest potentiation in contractile activity and Ca2+ transients was studied in the presence and absence of 40 µM streptomycin to block cation non-selective SACs. One-way ANOVA was used for statistical analysis and P < 0.05 was considered to indicate a significant difference between means.
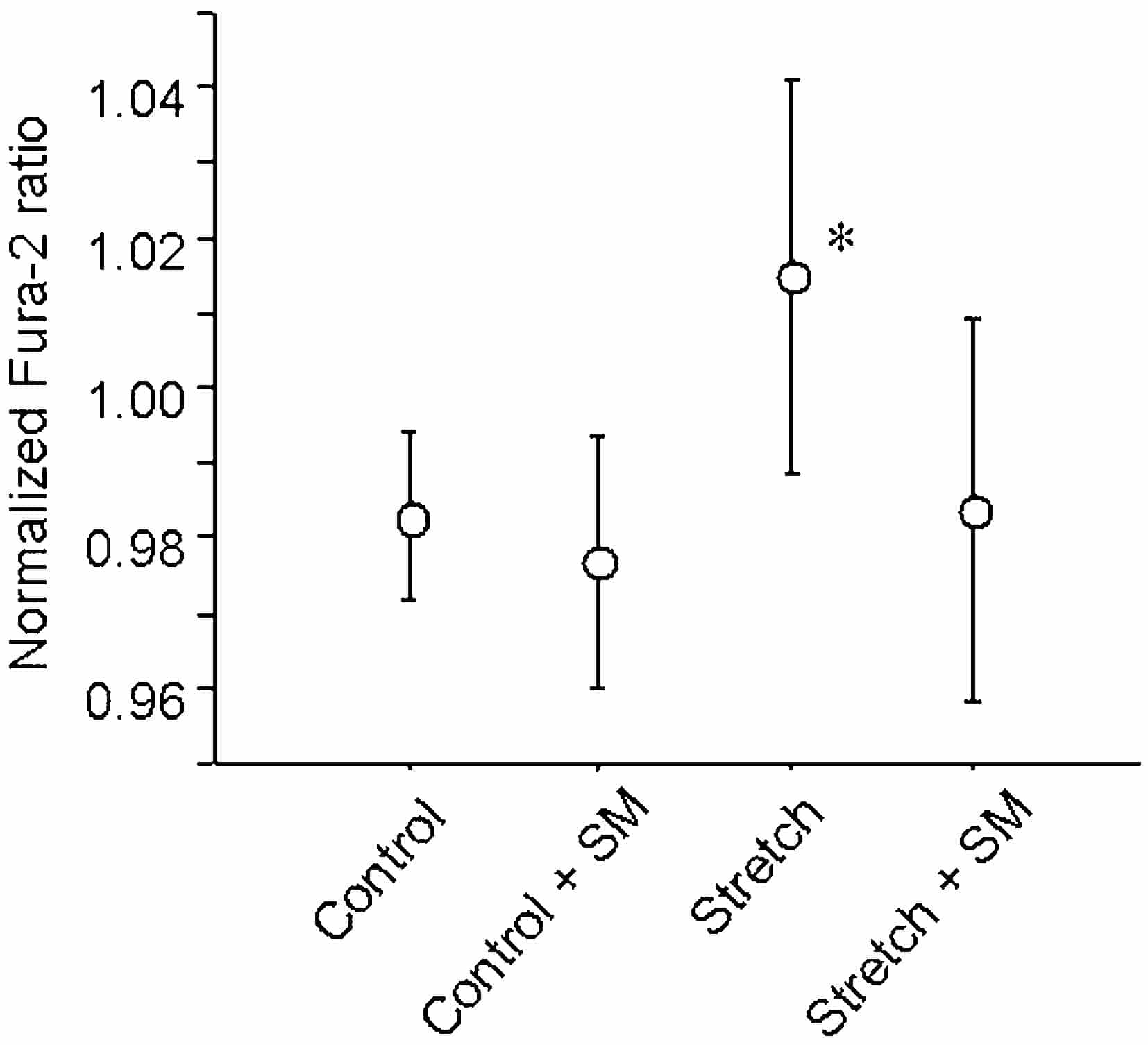
There was no detectable difference in diastolic [Ca2+]i (baseline Fura-2 signal at the end of rest period) between control and stretched states. Stretch caused a transient increase in post-rest potentiation of Ca2+ transients and contraction, which was significant after 5 s (Fig. 1). Longer rest periods were accompanied by an overall reduction of post-rest potentiation in both control and stretched cells. The initial increase in peak Ca2+ transient was prevented by application of streptomycin (Fig. 1).
The present results suggest that stretch transiently increases SR Ca2+ content in guinea-pig ventricular myocytes. This effect occurs in the absence of a notable increase in diastolic [Ca2+]i, and appears to be mediated via activation of streptomycin-sensitive SACs. The decrease in Ca2+ transient amplitudes after rest of 10 s or more could be attributable to Ca2+ leaks from SR and/or cell, and further studies are required to address this.
We thank Dr Michiel Helmes and Prof. David Eisner for help and advice. This work was supported by the BHF. P.K. is a Royal Society Research Fellow.