Although Ca2+ sparks have been recorded in a variety of cell types including smooth muscle cells, few confocal imaging systems are capable of recording sparks from the entire cell at frame rates in excess of 30 frames s-1 (f.p.s.), particularly whilst maintaining good resolution in terms of signal/noise and spatial definition. In the present study we have developed, manufactured and evaluated a novel back-illuminated electron multiplying charge coupled device (BI-EMCCD) camera (Coates et al. 2003), which allows us to detect Ca2+ sparks at frame rates in excess of 100 f.p.s.
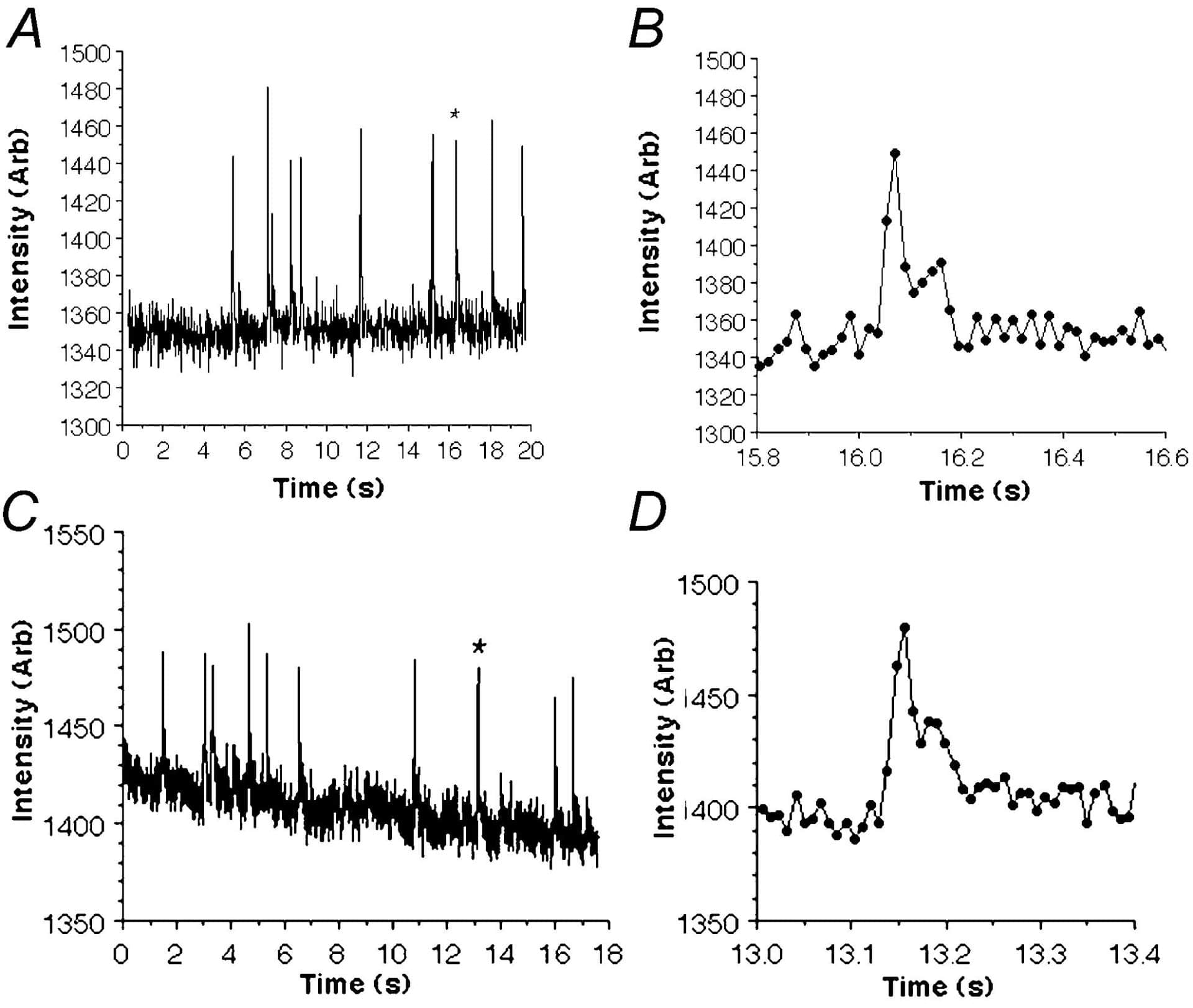
The EMCCD used contains a 512 X 512 pixel Marconi E2V chip (pixel size 16 µm) which achieves signal amplification through a unique electron multiplying structure built into the silicon, rather than the incorporation of an image intensifier tube. In contrast to intensified CCDs which typically possess quantum efficiencies (QE) of < 50 %, the EMCCD has a QE in excess of 90 % across a wider range of wavelengths and this makes it much better at detecting weak signals. For these experiments, guinea-pigs were humanely killed with pentobarbitone and freshly dispersed bladder smooth muscle cells were loaded with Fluo-4 AM (10 µM). An inverted Nikon TE300 Microscope with X 40 and X 100 objectives was combined with a spinning Nipkow disk arrangement (Visitech, UK). Using the EMCCD camera, Ca2+ sparks could be clearly resolved above background noise at speeds up to 31 f.p.s. (512 X 512 pixels). However when subregions of the CCD were selected, it was possible to reliably measure sparks at rates of 60 f.p.s. (512 X 200 pixels; 83 µm X 32 µm) and 120 f.p.s. (512 X 100 pixels; 83 µm X 16 µm). Figure 1 shows typical recordings from a 2.7 µm region of interest placed over the centre of a spark site and recorded at (A) 60 f.p.s. and (C) 113 f.p.s.. As panels B and D suggest, the differences in rise time and decay of individual sparks could be clearly resolved and support the idea that EMCCD cameras are suitable for detecting spontaneous calcium release across a large proportion of a smooth muscle cell with both high spatial and temporal resolution.
This work was supported by the Wellcome Trust and Action Research.