We have examined the relationship between the post-exercise stimulation of muscle protein synthesis and the activities of p70S6k and PKB, members of the anabolic signalling pathway.
Eight healthy males (25 ± 5 y, body mass index, 26 ± 3 kg.m-2 mean ± S.E.M.) were studied. Carrying 25 % body weight, the subjects stepped up onto a knee-high box with one leg and stepped down with the other at ~1 Hz for repeated bouts of 6, 3 and 3 min with 2 min rest between. Quadriceps muscle biopsies were taken from both legs (using 1 % lignocaine anaesthesia) before exercise and 3, 6 and 24 h post exercise. In the 2 h before each biopsy, subjects were fed intermittently 45 g essential amino acids and 135 g sucrose. We infused [1-13C]leucine (prime 1.0 mg kg-1; 0.8 mg kg-1 h-1) from rest to 6 h post-exercise and [113C]valine (prime 1.2 mg kg-1; 1.0 mg kg-1 h-1) between 21-24 h post exercise. Rates of myofibrillar and sarcoplasmic protein synthesis were measured by standard techniques using gas chromatography-combustion-isotope ratio mass spectrometry. Phosphorylation (activation) of p70S6k and PKB was quantified by Western blotting.
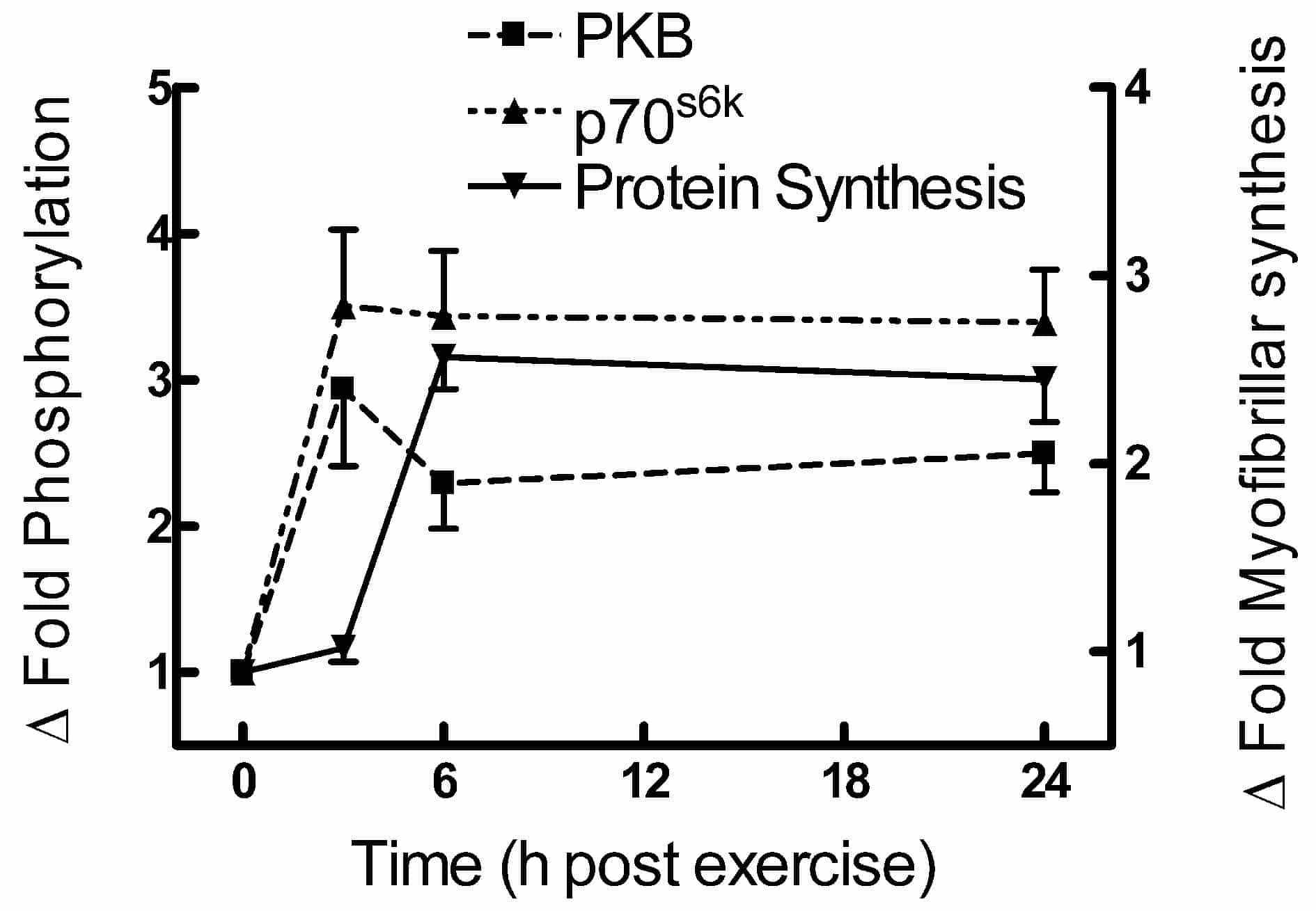
With this protocol there were no differences observed in any of the measured variables after lengthening or shortening contractions. PKB phosphorylation (Serine 473) rose to a maximum value (3.2 fold above baseline) at 3 h post exercise but was still significantly elevated at 24 h. However, phosphorylation of p70S6k (basal 23 % phosphorylation), increased to a constant value of ~3.5 fold above basal between 3 and 24 h. Muscle protein synthesis rose rapidly between 3 and 6 h and remained elevated for 24 h (basal myofibrillar and sarcoplasmic synthesis rates 0.042 ± 0.012 % h-1 and 0.061 ± 0.007 % h-1 respectively), the relative increase being 62 % greater for myofibrillar than sarcoplasmic protein at 24 h. The results are consistent with a sequence of events in which muscle contraction causes a sustained activation of PKB and p70S6k with a subsequent prolonged stimulation of protein synthesis. This is in contrast to the transient rise in PKB and p70S6k activity seen after acute exercise in the rat (Bolster et al. 2003).
This work was supported by Medical Research Council and the Wellcome Trust, UK.