Progesterone (produced by cumulus cells around the oocyte) evokes a biphasic Ca2+ influx in human spermatozoa and provides communication between the sperm and the oocyte. It is well established that stepped additions of progesterone, from zero dose to µM concentrations, to human spermatozoa evoke a transient Ca2+ influx followed by a sustained elevation (both of which are dependent upon extracellular Ca2+), and acrosome reaction. It is most unlikely that this stimulus profile occurs in vivo and therefore we have re-examined the role of progesterone in human gamete interaction by exposing spermatozoa to a slowly increasing progesterone gradient (3 nM-3 µM over 30 min), simulating the stimulus experienced by the sperm on approaching the oocyte.
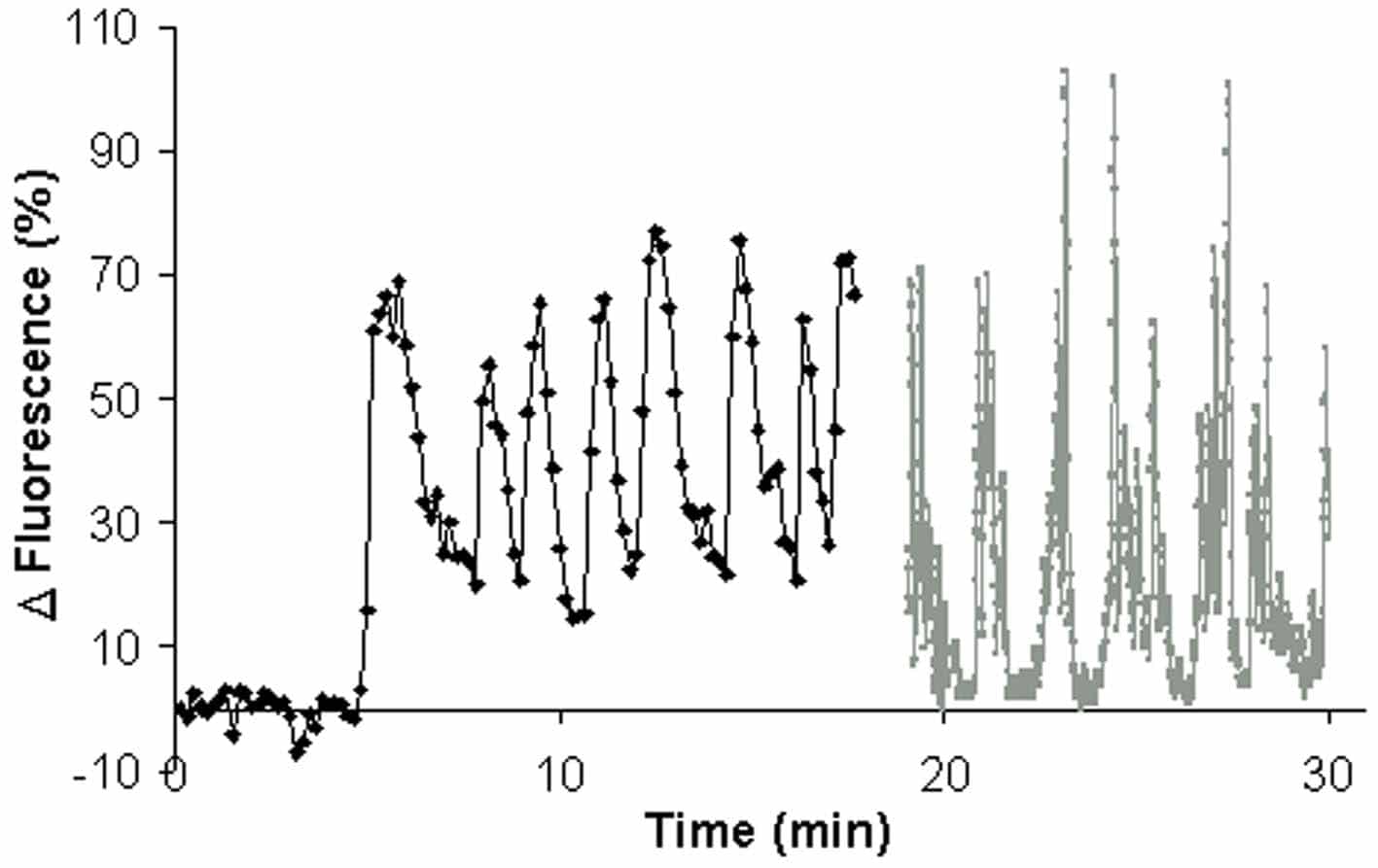
Spermatozoa were harvested by direct swim-up into sEBSS media with 0.3 % BSA and incubated for 6 h at 6 million cells ml-1. [Ca2+]i was monitored in cells loaded with Oregon Green-1 BAPTA and adhered to the base of a perfusion chamber (25°C). Upon application of progesterone nearly all cells generated a slow, steady increase in [Ca2+]i at the rear of the head. Latency of this response varied between cells, but 93.6 ± 1.6 % (S.E.M. 5 experiments;667 cells) of spermatozoa showed a significant response (P < 0.05; paired t) within 5 min (at nM concentrations of progesterone). Approximately 35 % of cells, after initiation of the gradient, show slow [Ca2+]i oscillations with a duration of 0.5-1 min and period 1.5-3 min. These cells show bursts of increased flagellar beating, during the oscillation peak (figure). Progesterone-induced oscillations are insensitive to thapsigargin (100 nM-5 µM) and modulators of the IP3 signalling pathway (neomycin [100 nM-20 µM], U73122 [10 µM]), but are arrested by the intracellular Ca2+ channel antagonist TMB-8 (300-500 µM).We propose that the effect of progesterone on [Ca2+]i in human spermatozoa in vivo may be markedly different to the well-characterised biphasic effect and that a significant aspect of this may be a periodic induction of increased motility controlled through store-derived oscillations in [Ca2+]i.
The research was carried out according to local ethical guidelines with the Birmingham Women’s Hospital (HFEA no. 0119). Donors gave informed consent. This work was supported by BBSRC and The Wellcome Trust.