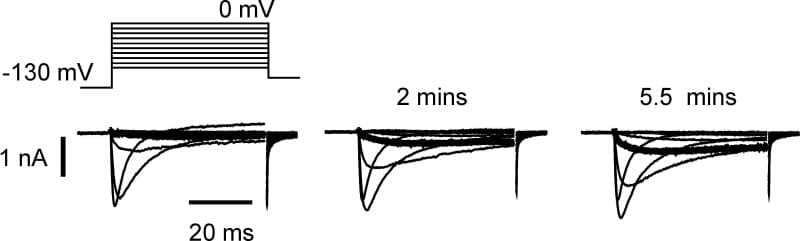
The persistent tetrodotoxin-resistant (TTX-r) Na+current, attributed to NaV1.9 (NaN), is expressed in peripheral nociceptive neurones (e.g. Fjell et al. 2000) and at unmyelinated nerve endings (Black & Waxman, 2002). It activates at membrane potentials near rest and can be dramatically up-regulated by at least one G-protein pathway, leading to depolarization, a more negative voltage-threshold for action potential induction and spontaneous activity in small diameter DRG neurones in culture (Baker et al. 2003). In order to clarify the mechanism of this functional up-regulation, experiments were performed on small (<25 μm apparent diameter) DRG neurones from P21 rat and adult WT and NaV1.8 null mouse, maintained in culture for 1 to 2 days. All voltage-clamp recordings were made in the presence of 250 nM external TTX. With 3 mM ATP internal, intracellular GTP-γ-S (500 μM) induced a near 300 % average increase in NaV1.9 (NaN) current amplitude in NaV1.8 null neurones over 5 minutes, an effect abolished by including the inhibitor PKC 19-36 in the internal solution (0.5 – 5 μM), n = 10 and 6, respectively; p = 0.035, 2-way repeated measures ANOVA. Incorporation of 1-oleoyl-2-acetyl-sn-glycerol (OAG) (25 μM) in an intracellular solution also containing GDP (500 μM) and ATP (3 mM), caused an increase in Na+ current recorded in WT neurones at -40 mV and more negative (Fig. 1), that did not occur in neurones with GDP and ATP only (n = 9 and 9, respectively; p < 0.03, Fisher exact test). In perforated-patch, voltage-clamp recordings from WT neurones, superfusion of dB-cAMP (1 mM) had no effect on the current, whereas exposure to phorbol 12-myristate 13-acetate (PMA; 1 μM) induced up-regulation in the same neurones (n = 2) consistent with the involvement of PKC but not PKA. Conversely, the major TTX-r Na+ current, NaV1.8, was unaffected by exposure to PMA (n = 3). Finally, superfusion of ATP up-regulated NaV1.9 (NaN) (n = 6), with an apparent Kd of 18.2 ± 4.6 μM (mean ± s.e.m., n = 3), possibly consistent with a P2Y receptor, Gq/11 coupled pathway. Sustained increases in nociceptor excitability caused by ATP might therefore be explained by the up-regulation of NaV1.9
King's College London (2005) J Physiol 565P, C83
Communications: Protein kinase C mediates up-regulation of NaV1.9 (NaN) in sensory neurones
Baker, Mark D;
1. Molecular Nociception Group, University College London, London, United Kingdom.
View other abstracts by:
Figure 1. OAG (25 μM) internal induces up-regulation of TTX-r Na‾1.8) is indicated by a thicker trace.
Where applicable, experiments conform with Society ethical requirements.