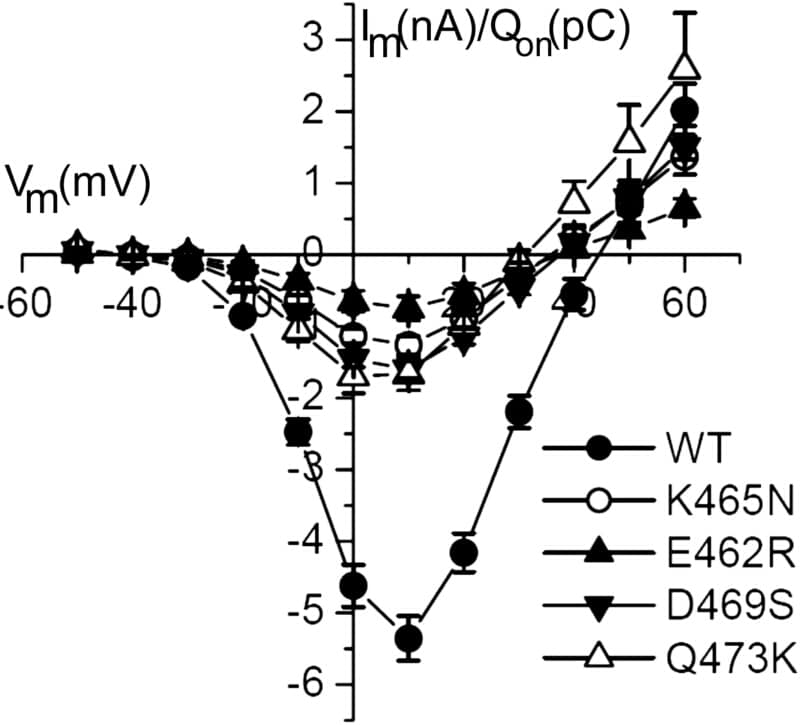
Voltage- Dependent Calcium Channels (VDCC) are hetero-multimeres composed by at least 3 subunits: the pore forming subunit α1 and auxiliary subunits α2/δ and β. The β subunit binds to α1 at a conserved sequence of 18 amino acids in the loop joining the first a second repeat (AID; 1) In this work we show that non-conserved amino acids within the AID motif alter channel function without effect on expression. Charged amino acids in the rat CaV1.2 AID motif were either reversed or neutralized (E462R, K465N, D469S and Q473K). When co-expressed in Xenopus oocytes cardiac with the bβ-subunit (β2a), peak inward currents normalized by charge movement were reduced by 80 to 90% for all mutants while charge movement itself remain unchanged suggesting that regulation of expression was not affected. These mutations do not appear to prevent α1-β interaction since voltage-dependence of activation is left-shifted by about 40 mV with co-expression of β2a. Single channel conductance remained constant but the channel probability of being open (Po) was greatly reduced. Further studies on E462R and K465N revealed that decreases in channel Po occur through different mechanisms in each case. For E462R, repeated depolarisations (1 Hz) to 0 mV from a holding potential of -70 mV yield a large number of sweeps without channel activity (80±4% of null sweeps for E462R compared to 39±7% found in wild type CaV1.2) while activity within active sweeps is not affected. Surprisingly, reduction in the Po by a neutralization of a Lysine three residues downs stream within AID (K465N), correlates with sweeps of activity with short burst of openings (1.3±0.5 ms) without changes in the fraction of null sweeps (35±13%). It appears then that altering the α1-β interaction surface as in E462R mutation, promotes a silent mode while in K465N, the low Po mode become prevalent. These results support the view that the β subunit regulates function by tuning dwell times among different gating modes.
King's College London (2005) J Physiol 565P, PC74
Communications: Charged amino acids within the alpha interaction domain (AID) of Voltage-Gated Calcium Channels regulate single channel behaviour
Gonzalez-Gutierrez, Giovanni ; Hidalgo, Patricia ; Neely, Alan ;
1. Centro de Neurociencias de Valparaiso, U. de Valparaiso, Valparaiso, Chile. 2. Institut fur Physiologie, RWTH Aachen, Aachen, Germany.
View other abstracts by:
Figure 1: IV curves for the different mutants of alpha1C. Im (nA) was normalized by charge movement (Qon) and Qon (pC) was measured at the onset of a depolarisation pulse to the current reversal potential (2)
Where applicable, experiments conform with Society ethical requirements.