Shaker-type voltage-gated K+ (Kv1.x) channels are composed of a tetramer of pore-forming α subunits which can associate with regulatory proteins such as the Kvβ subunits to form an α4β4 complex. Cytosolic Kvβ subunits, which interact with Kvα subunits through binding to the T1 domain, have been demonstrated to modify the kinetics of Kv channels. Thus understanding Kvα-β interactions, may offer a novel mechanism to exploit the function of Kv channels for therapeutic benefit. The aim of this study was to develop a robust and sensitive assay to determine the nature of the interaction between rKvα1.1 T1 domains and rKv β1 core proteins to allow subsequent identification of compounds which modulate this interaction. rKvα 1.1 T1 domains and rKv β1 core proteins were expressed in E. coli as specific fusion proteins, incorporating biotin carboxyl carrier protein as an affinity tag to the rKv β1 core protein. Purified rKvα 1.1 T1 domains were labelled with Cy3 dye. rKv β1 core proteins were immobilised through biotin-streptavidin interactions in a microwell format, with rKvα 1.1 T1 domains titrated into the microwells to form the basis of a fluorescent-readout protein-protein interaction assay. In order to optimise the assay, binding kinetics of the interaction between rKvα 1.1 T1 domains and rKv β1 core proteins were defined (analyses derived from Davies et al. 1999), see Table 1. The characterisation of rKvα 1.1 T1 domain – rKv β1 core protein interactions enabled construction of a cell-free displacement assay based in a microplate format. Assay parameters were defined following optimisation of the assay for a 96 well plate format. Throughput in this semi-automated process is 200 compounds per day with assay plate coefficient of variation less than 10% (n > 20). A compound series was identified that modulated the interaction between rKv α 1.1 T1 domains and rKv β1 core proteins (see Table 2 for the compound series). In summary, determination of the binding kinetics of the interaction between rKv α1.1 T1 domains and rKv β1 core proteins allowed the construction of a robust, fully scaleable assay that was used to identify compounds that modulate this interaction. Compounds identified within this assay may serve to modulate Kv channel kinetics through a novel mode of action.
University of Bristol (2005) J Physiol 567P, C89
Oral Communications: In vitro assay for identifying modulators of Kv channel α-β subunit interactions
Stafford, Simon; Ginham, Rachel; Davies, Andrew R. L.; Lawton, Geoff; Boden, Phil R.; Kozlowski, Roland Z.;
1. Lectus Therapeutics Limited, PO Box 2299, Bristol, BS99 5YG, United Kingdom. 2. Department of Pharmacology, School of Medical Sciences, University of Bristol, Bristol, BS8 1TD, United Kingdom.
View other abstracts by:
Table 1. Binding parameters for interactions between of rKv1.1 T1 domain with rKv β1 core protein (n = 3).
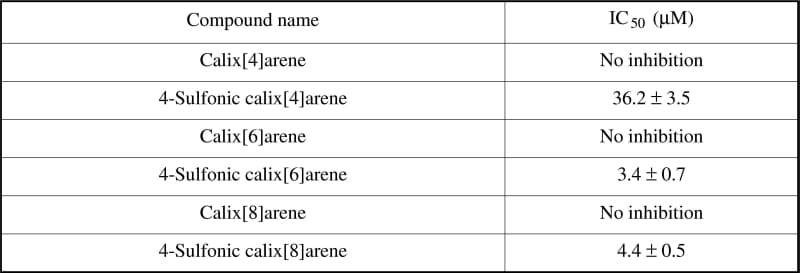
Table 2. Compound series demonstrated to modulate the interaction of the rKv α/β subunits in the microplate assay format.
Where applicable, experiments conform with Society ethical requirements.