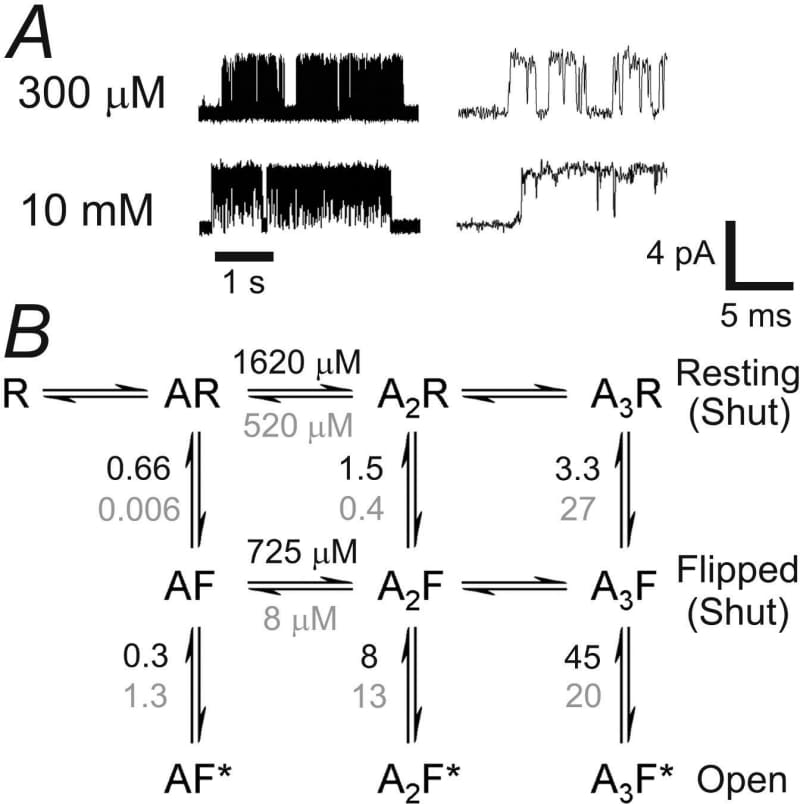
In the spinal cord and brainstem, fast inhibitory transmission via glycine receptors controls muscle tone and locomotion. A loss-of-function mutation in the glycine receptor α1 subunit, A52S, gives rise to spasmodic, a mouse with a phenotype that resembles human hyperekplexia (startle disease) (1). Synaptic currents are smaller, and decay faster, than normal (2). We expressed rat heteromeric (α1β) receptors with this mutation in human embryonic kidney 293 cells, and recorded single-channel data in the cell-attached configuration (see Fig. 1). The maximum Popen for A52S receptors was 97 ± 1% (n = 2-5 patches per point), similar to wild type (3). The EC50 for Popen was increased by a factor of 5, from 60 μM glycine (3) for wild-type receptors to 326 ± 9 μM (n = 2-5 as before), and the Hill slope was reduced from 3.2 (3) to 1.9 ± 0.1. Several reaction schemes were fitted to data at three concentrations of glycine (four sets of simultaneous maximum-likelihood fits with HJCFIT (3)). Good fits were obtained using mechanisms in which three glycine molecules could bind to the receptor, and where an extra shut state was included (in addition to the resting and open states) at each level of liganding. Gating efficacy was similar to that of wild-type receptors (the fully-liganded efficacy was about 40 (3)). The apparent strong cooperativity in binding of successive glycine molecules to shut channels in wild type (3) was much less obvious in A52S receptors. Good fits could be obtained with the affinity for all binding reactions fixed at K = 1000 μM. The ‘flip’ mechanism (3) (see Fig. 1) postulates a physical interpretation of the extra shut states, and a mechanism for the apparent cooperativity. Fits for A52S receptors showed a difference in glycine affinity between the resting and flipped forms of the receptor much smaller than for wild-type receptors (KR/KF ≈ 2 for A52S, compared to 65 for wild type (3)). We conclude that the increase in glycine EC50 in the A52S mutation (which is unlikely to be in the binding site) results roughly equally from a decrease in resting affinity, and from the weakening of the conformational change between shut forms of the receptor, so reducing the length of channel activations.
University of Bristol (2005) J Physiol 567P, C93
Oral Communications: Single channel study of the spasmodic mutation (A52S) in the glycine receptor
Plested, Andrew; Groot-Kormelink, Paul; Sivilotti, Lucia G; Colquhoun, David;
1. Pharmacology, UCL , London, United Kingdom.
View other abstracts by:
Figure 1. A typical single A52S glycine receptor activations at 300 µM and 10 mM glycine. B the \"flip\" model. Equilibrium constants (calculated for the fitted rate constants vertical ones are the downward rate divided by the upward) are shown for A52S (black) and wild-type (grey). The mutation reduces the affinity of glycine for the flipped state much more than for the resting state so the increase in glycine affinity after flipping is much smaller.
Where applicable, experiments conform with Society ethical requirements.