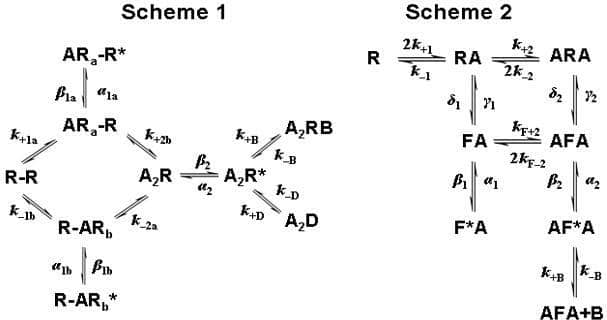
We investigated the effect on receptor function of a naturally occurring congenital myasthenic syndrome mutation S268F in human acetylcholine (ACh) receptor δ subunit. Single channel currents were recorded in cell-attached configuration (at —100 mV transmembrane potential) from HEK293 cells transfected with wild type ACh receptor α, β, ε and δ (or δS268F) subunits. A resolution of 15-25 μs was imposed retrospectively. All means are given ± their standard deviation. The mutant receptors produced longer bursts of openings than the wild type receptors, as expected since this mutation slows endplate current decay. EC50s of 5.3 ± 0.3 μM (wild type) and 0.23 ± 0.01 μM (mutant; n = 3-5 patches per point) were found by fitting the Hill equation to single-channel Popen curves. Several mechanisms were fitted by maximising the likelihood of the entire sequence of open and shut time periods, with exact allowance for missed brief events (program HJCFIT; Colquhoun et al. 2003). Records obtained at several different ACh concentrations were fitted simultaneously. In order to get good fits, it was essential to include an extra shut state, either distal to the open state (D in scheme 1) or as a proximal ′flip′ state (F in scheme 2) (Colquhoun et al. 2003; Burzomato et al. 2004). For Scheme 2 the gating equilibrium constant or efficacy, E = β/α, was 34 ± 3 and 54 ± 1 (n = 4 sets) in wild type and mutant receptor, respectively. ACh dissociation rate from diliganded receptor was about 10 times slower for the mutant (1000 ± 1000 s-1; n = 4 sets) than for wild type (9000 ± 6000 s-1; n = 4 sets). Dissociation rate from the ′flipped′ conformation (F) in scheme 2 was about 20 times slower for the mutant (300 ± 300 s-1; n = 4 sets) than for wild type (6000 ± 4000 s-1; n = 4 sets,). The last dissociation rate appears to be major contributor to the slowing of the endplate current decay. Other rate constants were not dramatically different between wild type and mutant receptors. The fitted rates predict that δS268F mutation slows the endplate current decay about 15-fold and decreases EC50 about 20 times. These effects appear to result mostly from a reduction in ACh dissociation rates.
University of Bristol (2005) J Physiol 567P, PC144
Poster Communications: Effect of slow channel congenital myasthenic syndrome mutation δS268F on human acetylcholine receptor function
Lape, Remigijus; Colquhoun, David;
1. Pharmacology, University College London, London, United Kingdom.
View other abstracts by:
Figure 1. Scheme 1 has a short-lived €˜desensitised€™ state distal to the open state. Scheme 2 includes a change in conformation (\"flip\") before opening. Both schemes include channel block by ACh. R = shut receptor R* or F* = open channel A = ACh B = blocked channel. F = flipped (but shut) conformation.
Where applicable, experiments conform with Society ethical requirements.