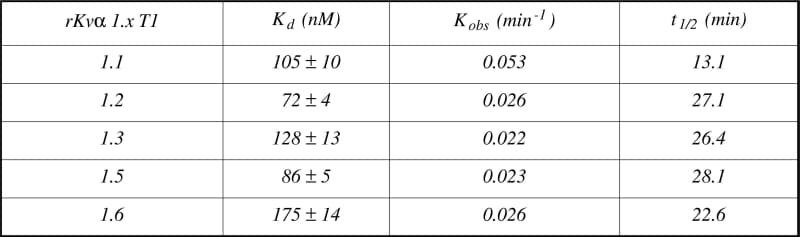
Functional voltage-gated K+ (Kv) channels exist as homomeric or heteromeric tetramers of Kv α subunits that form the channel ‘pore’. The kinetics of Shaker-type Kv (Kvα 1.x) channel currents can be specifically modulated by accessory proteins know as Kvβ subunits that bind to Kv1.x subunit counterparts through N-terminal region known as the ‘T1’ domain (Gulbis et al. 2000). Since Kvβ subunits have been demonstrated to differentially modulate Kvα 1.x channel kinetics (Sewing et al. 1996), this study aimed to determine the binding kinetics between Kvα 1.1, Kvα 1.2, Kvα 1.3, Kvα 1.5 & Kvα 1.6 T1 domains to the Kv β1 ‘core’ protein. rKv β1 core proteins were cloned from whole brain tissue samples obtained from humanely killed rats, and a sequence encoding an affinity tag (biotin carboxyl carrier protein) incorporated into the cDNA. rKv β1 core proteins were subsequently expressed in E. coli and immobilised onto streptavidin substrates via the affinity tag, through a biotin-streptavidin interaction. rKvα 1.x T1 domains were cloned from rat whole brain cDNA samples, expressed in E. coli, purified and labelled with Cy3 dye. Binding studies were conducted by quantitative fluorescent measurement of Cy-3 labelled rKv 1.x T1 αdomain binding to immobilised rKv β1 core proteins. Analyses of the binding parameters were carried out using conventional methodologies (Davies et al. 1999) and are summarised in Table 1. These results show that rKv α1.x T1 domains display differential binding kinetics to immobilised rKv β1 subunits as demonstrated by the differences in equilibrium dissociation constants and rates of association. The differential binding affinity of rKvα 1.x T1 domains to rKv β1 subunits may underlie the differential functional modulation of native rKv 1.x currents by rKv β1 subunit accessory proteins and ultimately facilitate the characterisation of a novel mode of modulating Kv1.x channels in a subtype selective manner.
University of Bristol (2005) J Physiol 567P, PC156
Poster Communications: Differential binding kinetics between Shaker-related Kv1 channels and the Kvβ1 regulatory protein
Ginham, Rachel; Stafford, Simon; Davies, Andrew R. L.; Kanumilli, Srinivisan; Kozlowski, Roland Z.;
1. Department of Pharmacology, School of Medical Sciences, University of Bristol, Bristol, BS8 1TD, United Kingdom.
View other abstracts by:
Table 1. Binding parameters of rKvα 1.x T1 domains to immobilised Kv β1 core proteins (n = 3). Kd equilibrium dissociation constant; Kobs observed association rate constant; t1/2 amount of time required for the ligand to occupy 50% of the binding sites.
Where applicable, experiments conform with Society ethical requirements.