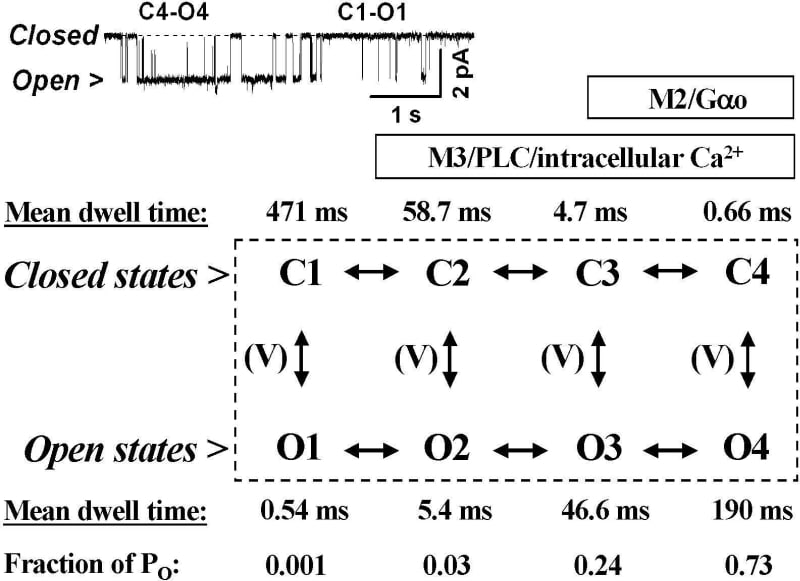
Cholinergic excitation of the gastrointestinal smooth muscles is mediated by cation channels via a synergistic action of the predominant M2 (~80%) and minor M3 (~20%) muscarinic receptors. Cation channel of 57±7 pS conductance (means±S.E.M., n=55) carries most of the muscarinic inward current, mICAT (Zholos et al. 2004a). The gating signals apart from [Ca2+]i elevation and membrane depolarization include Pertussis toxin sensitive M2-mediated activation of Gαo protein (Yan et al. 2003) and concurrent M3-dependent activation of phospholipase C, PLC. mICAT appears to be directly activated by the M2 receptor since adenylyl cyclase is not involved while the nature of the M3 receptor action remains enigmatic. Apart from [Ca2+]i-dependent potentiation of mICAT due to InsP3-induced Ca2+ release it also includes another important but yet unidentified process, in which PLC products, diacylglycerols and InsP3, as well as Ca2+ store depletion are not involved (Zholos et al. 2004b). Experiments were performed on single collagenase-dispersed myocytes isolated from the small intestine of humanely killed adult guinea-pigs using a combination of patch-clamp recordings, confocal [Ca2+]i imaging (0.1 mM fluo-3 signal) and flash photolysis of ‘caged’ InsP3 (30 μM in the pipette) or Ca2+ (5 mM NP-EGTA/3.8 mM Ca2+). We aimed to relate the multiple signalling pathways to the channel mechanism. The 60 pS channel in outside-out patches exposed to 50 μM carbachol externally or 200 μM GTPγS internally showed transitions between 8 states with strong correlations between the dwell times of adjacent shut and open states. Thus, four pairs of connected states have been identified which showed voltage-dependent vertical transitions in each pair (Fig. 1, note that mean dwell times and fractional contribution of each open state to the channel open probability, PO, are indicated). Prominent regular cycles of PO occurred because of a variable number of long openings between consecutive long shuttings, that is due to the horizontal transitions between the ‘low-PO mode’ in C1-O1 and the ‘high-PO mode’ in C4-O4 states. These probably occurred in a ligand-dependent manner since horizontal redistribution between the channel states was voltage independent. In Cs+-rich (125 mM) divalent cation-free solution which maximizes mICAT spontaneous cation current, sICAT, was revealed with voltage dependence similar to mICAT (e.g. U-shaped I-V curve at negative potentials). Its amplitude was 192±9 pA at −40 mV increasing to 1590±209 pA at 80 mV (n=8). sICAT was insensitive to atropine (50 nM) suggesting that mAChR constitutive activity was not involved. sICAT relaxations during steps from −40 to −120 mV were fast consistent with the O1 mean open time; in 3 out of 8 cells a slower component with τ=4.7±1.2 ms was also detected. Photorelease of Ca2+ significantly potentiated this current in a voltage-dependent manner (760±120 and 4860±137 pA at −40 and 80 mV, respectively), while slower relaxations with the time constant in the range 2.8-9.5 ms (7.5±0.4 ms; n=18) became apparent in the macroscopic kinetics — the values typical for the O2 microstate. Carbachol application (10 μM) initiated Ca2+ waves and mICAT, the latter occurred with a latency of 229±55 ms (n=7) and peak lagging by 1.22±0.11 s (n=17) compared to the fluo-3 signal. However, at later times mICAT mirrored changes in [Ca2+]i induced by an additional photorelease of InsP3 (time-to-peak 279±25 and 298±23 ms, respectively; n=7). Moreover, 5 mM caffeine-induced Ca2+ release was totally inefficient in modulating mICAT while photorelease of Ca2+ was poorly efficient compared to the InsP3-mediated modulation of mICAT. This suggested an important synergy between InsP3 and Ca2+-dependent activation of the channel although InsP3 alone was inefficient when [Ca2+]i was clamped at 100 nM using 10 mM BAPTA. Our results suggest that in the absence of ligands intrinsic voltage-dependent channel gating occurs in the ‘low-PO‘ C1-O1 mode. The ‘high PO‘ C4-O4 mode is probably dominated by the activated Gαo interaction with the channel since Pertussis toxin nearly abolishes mICAT. Intracellular Ca2+ and the M3/PLC/InsP3 system may therefore shift the gating through the intermediate states (Fig. 1).
University of Oxford (2005) J Physiol 568P, SA5
Research Symposium: Muscarinic cation current of the ileum: signalling events and channel gating
Zholos, Alexander V; Gordienko, Dmitri V; Tsvilovskyy, Vladimir V; Bolton, Thomas B;
1. Molecular Pharmacology, Bogomoletz Institute of Physiology, Kiev, Ukraine. 2. Basic Medical Sciences, St George's, University of London, London, United Kingdom.
View other abstracts by:
Fig. 1. Channel mechanism with mean parameters indicated. The inset shows gating typical for the C4-O4 and C1-O1 transitions.
Where applicable, experiments conform with Society ethical requirements.