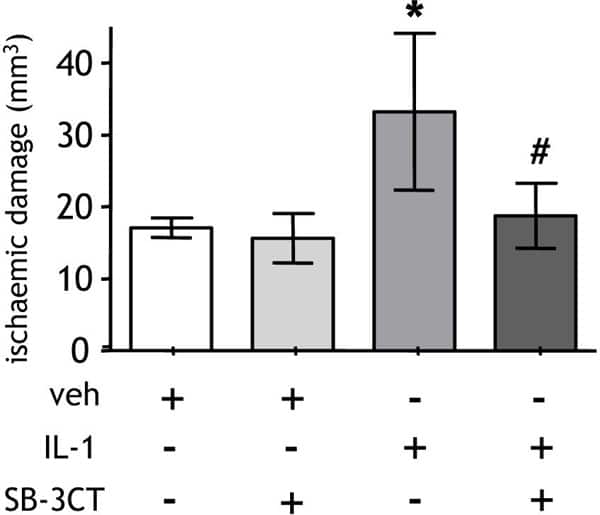
Growing evidence suggests that systemic inflammation modulates the response to acute brain injury and the progression of neurodegenerative diseases (Emsley & Tyrrell, 2002; Perry, 2004). Peripheral inflammatory stimuli, such as infection, increase the risk of stroke and are associated with poorer outcome but the mechanisms are poorly defined (Smeeth et al. 2004; Palasik et al. 2005). We recently demonstrated that a systemic inflammatory challenge exacerbates brain damage after experimental stroke and that the cytokine interleukin-1 (IL-1) is a critical mediator in this paradigm (McColl et al. 2007). In the current study, we further investigated the mechanisms involved. We tested the hypothesis that a systemic inflammatory challenge exacerbates brain damage after experimental stroke by potentiating neutrophil responses and investigated the role of matrix-metalloproteinase-9 (MMP9) in this paradigm. Focal cerebral ischaemia was induced in C57Bl/6J mice by transient (30min) middle cerebral artery occlusion (under isoflurane anaesthesia (3.5% in 30% O2-70% N2O)). Systemic inflammation was induced by recombinant IL-1β challenge (i.p.) and the volume of ischaemic damage and neurological deficit determined. Cerebral neutrophil accumulation, MMP9 immunoreactivity and gelatinolytic activity were assessed 8h and 24h after MCAo. The role of MMP9 was tested by co-administration of an MMP9 inhibitor (SB-3CT) with IL-1β. Systemic administration of recombinant IL-1β significantly exacerbated ischaemic brain damage and neurological deficit and potentiated circulating neutrophil counts and cortical neutrophil infiltration. Neutropenia attenuated the IL-1β-induced exacerbation of ischaemic damage. IL-1β increased cortical MMP9 immunoreactivity and gelatinolytic activity. MMP9 immunoreactivity was observed in neutrophils and blood vessels whereas gelatinolytic activity was observed primarily in the cerebral vasculature. Co-administration of the MMP9 inhibitor, SB-3CT, attenuated the IL-1β-mediated exacerbation of ischaemic damage. These data show the detrimental effects of a systemic inflammatory challenge on experimental stroke outcome and indicate key roles for neutrophils and MMP9 in this paradigm. These mechanisms may underlie the poorer outcome in stroke patients presenting with infection and may have implications for stroke aetiology in general since most stroke patients will present with pre-existing systemic inflammation linked to co-morbidities such as atherosclerosis and heart disease.
Life Sciences 2007 (2007) Proc Life Sciences, C38
Research Symposium: Systemic inflammatory stimulus exacerbates brain damage after experimental stroke via a matrix metalloproteinase-9-dependent mechanism that targets the neurovascular unit
B. W. McColl1, S. M. Allan1, N. J. Rothwell1
1. University of Manchester, Manchester, United Kingdom.
View other abstracts by:
Figure1. Systemic challenge with recombinant IL-1β at the onset of MCAo significantly increased the extent of brain damage and this was attenuated by co-administration of SB-3CT.
Where applicable, experiments conform with Society ethical requirements.