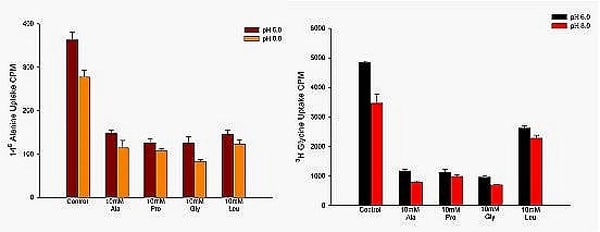
We have recently shown a novel role for an amino transporter, Path, in signalling in the fruit fly Drosophila melanogaster (Goberdhan et al 2005). This is a member of the proton-coupled amino acid transporter PAT family, with highly unusual functional properties. We describe here preliminary experiments using a cell line designed to complement our functional understanding of both this group as well as of the CD98 related group of amino acid transporters in this model organism. Figure 1 shows that S2 cells, known to express at least two (CG13384 and Path) of the 11 PAT genes found in the Drosophila genome, express readily-detectable transport properties expected for proton-coupled transporters (stimulation by acidification under sodium-free conditions; inhibition by the characteristic substrates Ala, Pro, Gly); however additionally the differing potency for inhibition by Leucine of the transport of the substrates Ala and Gly shows that more than a single PAT transporter must be involved. We have also shown that the system-L specific-substrate BCH is a potent inhibitor (under sodium-free conditions) of radiolabelled leucine influx into S2 cells. This means that these cells must express both the heavy chain (CD98 homologue) as well as the appropriate light chain (since system L is a heterodimeric transporter). S2 cells express a putative CD98 heavy chain homologue (CG2791) as well as five possible light chains. Knock-down of CG2791 using a standard RNAi protocol reduces system L-mediated leucine influx specifically. We conclude that S2 cells express endogenous transporters that will allow transport/signalling roles of both these families (PATs and CD98-related transporters) to be studied systematically in vitro while also exploiting the unrivalled genetic tractability of the fruitfly to understand their functional significance in vivo.
Life Sciences 2007 (2007) Proc Life Sciences, C43
Research Symposium: Amino acid transporters and cell signalling in a Drosophila cell line (Schneider (S2) cells)
B. Reynolds1, R. Laynes1, M. Ögmundsdóttir1, C. Boyd1, D. Goberdham1
1. Department of Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom.
View other abstracts by:
Figure 1: Influx of radiolabelled L-alanine (left) and of glycine (right) into S2 cells under sodium-free conditions: the external pH was respectively 6.0 or 8.0. Inhibition by 10mM unlabelled alanine proline glycine and leucine is shown. Note the pH dependence of uptake; and the different pattern of inhibition by leucine for the two substrates.
Where applicable, experiments conform with Society ethical requirements.